alleen voor onderzoeksdoeleinden
TMZ(Temozolomide) Alkylating Agent
Cat.nr.S1237
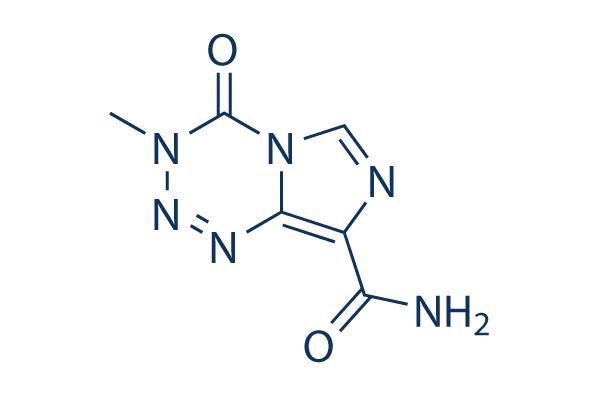
Chemische structuur
Molecuulgewicht: 194.15
Kwaliteitscontrole
| Gerelateerde doelwitten | HDAC PARP ATM/ATR DNA-PK WRN Topoisomerase PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Overig DNA/RNA Synthesis Inhibitoren | CX-5461 (Pidnarulex) B02 SCR7 Favipiravir (T-705) EED226 RK-33 BMH-21 Triapine (3-AP) Carmofur YK-4-279 |
Celkweek, behandeling & werkzame concentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| Kelly | Growth Inhibition Assay | 48 h | IC50=139.20 ± 5.95 μM | 25960282 | ||
| KellyCis83 | Growth Inhibition Assay | 48 h | IC50=251.00 ± 15.75 μM | 25960282 | ||
| SK-N-AS | Growth Inhibition Assay | 48 h | IC50=227.70 ± 22.15 μM | 25960282 | ||
| SK-N-ASCis24 | Growth Inhibition Assay | 48 h | IC50=480.60 ± 101.15 μM | 25960282 | ||
| CHP-212 | Growth Inhibition Assay | 48 h | IC50=7.97 ± 0.69 μM | 25960282 | ||
| CHP-212Cis100 | Growth Inhibition Assay | 48 h | IC50=9.55 ± 0.88 μM | 25960282 | ||
| U87 | Function Assay | 100 μM | 24-72 h | induces DcR1 expression | 25808868 | |
| LN229 | Growth Inhibition Assay | 0-50 μM | IC50=16 μM | 25750273 | ||
| TR-LN229 | Growth Inhibition Assay | 0-50 μM | IC50=77 μM | 25750273 | ||
| U87 | Apoptosis Assay | 0–200 µM | 24 h | enhances CQ induced apoptosis | 25681668 | |
| U251MG | Apoptosis Assay | 100 μM | 48 h | PBS | induces apoptosis | 25680464 |
| U87MG | Apoptosis Assay | 100 μM | 48 h | PBS | induces apoptosis | 25680464 |
| U87 | Growth Inhibition Assay | 50-350 μM | 48 h | inhibits cell growth slightly | 25554223 | |
| U118 | Growth Inhibition Assay | 50-350 μM | 48 h | inhibits cell growth slightly | 25554223 | |
| U87 | Function Assay | 250/350 μM | 48 h | enhances TMX-induced p-PKC-pan decrease | 25554223 | |
| U118 | Function Assay | 250/350 μM | 48 h | enhances TMX-induced p-PKC-pan decrease | 25554223 | |
| U87 | Growth Inhibition Assay | 250/350 μM | 48 h | increases the percentage of cells in S and G2/M cotreated with TMX | 25554223 | |
| A375 | Growth Inhibition Assay | 48 h | IC50=265 μM | 25524552 | ||
| A2058 | Growth Inhibition Assay | 48 h | IC50=12 μM | 25524552 | ||
| M238 | Growth Inhibition Assay | 48 h | IC50=40 μM | 25524552 | ||
| M249 | Growth Inhibition Assay | 48 h | IC50=254 μM | 25524552 | ||
| M21 | Growth Inhibition Assay | 48 h | IC50=221 μM | 25524552 | ||
| U251 | Cytotoxity Assay | 20 μM | 48 h | reduceds the percentages of colonies formed | 25434381 | |
| LN229 | Cytotoxity Assay | 20 μM | 48 h | reduceds the percentages of colonies formed | 25434381 | |
| U373MG-LUC | Growth Inhibition Assay | 72 h | IC50>600 μM | 25431953 | ||
| U87 | Growth Inhibition Assay | 25-200 μM | 48 h | inhibits cell growth in a dose-dependent manner | 25400745 | |
| U251 | Growth Inhibition Assay | 25-200 μM | 48 h | inhibits cell growth in a dose-dependent manner | 25400745 | |
| U251 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U373 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U343 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U87MG-luc2 | Growth Inhibition Assay | 100-400 μM | 72/96 h | the anti-proliferative effect can be enhanced by gossypol enhanced | 25375271 | |
| U87 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| U251 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| A172 | Function Assay | 200 μM | 48 h | increases BRCC3 mRNA expression | 25337721 | |
| U251 | Function Assay | 200 μM | 48 h | increases the expression of BRCA1, BRCA2, RAD51 and FANCD2 | 25337721 | |
| A172 | Function Assay | 200 μM | 48 h | increases the expression of BRCA1, BRCA2, RAD51 and FANCD2 | 25337721 | |
| U87 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| U251 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| A172 | Function Assay | 200 μM | 24/72/120 h | increases γH2AX foci formation time-dependently | 25337721 | |
| SNB19V | Growth Inhibition Assay | 7 d | DMSO | GI50=35.7±12 μM | 25277441 | |
| SNB19M | Growth Inhibition Assay | 7 d | DMSO | GI50=469.9±88 μM | 25277441 | |
| SNB19VR | Growth Inhibition Assay | 7 d | DMSO | GI50=280.2±18 μM | 25277441 | |
| U373V | Growth Inhibition Assay | 7 d | DMSO | GI50=68.0±32 μM | 25277441 | |
| U373M | Growth Inhibition Assay | 7 d | DMSO | GI50=368.7±86 μM | 25277441 | |
| U373VR | Growth Inhibition Assay | 7 d | DMSO | GI50=288.8±33 μM | 25277441 | |
| U87MG | Growth Inhibition Assay | 7 d | DMSO | GI50=38.3±20 μM | 25277441 | |
| HCT116 | Growth Inhibition Assay | 7 d | DMSO | GI50=579.9±32 μM | 25277441 | |
| DLD1 | Growth Inhibition Assay | 7 d | DMSO | GI50=501.4±93 μM | 25277441 | |
| MRC5 | Growth Inhibition Assay | 7 d | DMSO | GI50=449.4±8 μM | 25277441 | |
| SNB19V | Function Assay | 100 μM TMZ | 0-72 h | increases γH2AX expression between 16 and 72 h | 25277441 | |
| T98G | Growth Inhibition Assay | 5/10/15 μM | 24 h | induces cell death dose-dependently after concomitant-temozolomide with NPe6-PDT | 25262961 | |
| U251 | Growth Inhibition Assay | 5/10/15 μM | 24 h | induces cell death dose-dependently after concomitant-temozolomide with NPe6-PDT | 25262961 | |
| T98G | Function Assay | 15 μM | 24 h | increases DNA-fragmentation in NPe6-PDT treated glioma cells | 25262961 | |
| U251 | Function Assay | 15 μM | 24 h | increases DNA-fragmentation in NPe6-PDT treated glioma cells | 25262961 | |
| U-87 MG | Growth Inhibition Assay | 72 h | IC50=0.93 mM | 25245332 | ||
| U-118 MG | Growth Inhibition Assay | 72 h | IC50=1.05 mM | 25245332 | ||
| U87 | Growth Inhibition Assay | 24 h | IC50=260.34 μM | 25173233 | ||
| U87 GSLCs | Growth Inhibition Assay | 24 h | IC50=766.11 μM | 25173233 | ||
| U87MG | Growth Inhibition Assay | 72 h | IC50=15.625 μM | 25050915 | ||
| U251 | Growth Inhibition Assay | 100-400 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| U87 | Growth Inhibition Assay | 100-400 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-231-br | Growth Inhibition Assay | 0-10 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| HCC-1937 | Growth Inhibition Assay | 0-300 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-231 | Growth Inhibition Assay | 0-40 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MDA-MB-468 | Growth Inhibition Assay | 0-500 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| T47D | Growth Inhibition Assay | 0-100 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| MCF7 | Growth Inhibition Assay | 0-1000 μM | 48 h | DMSO | inhibits cell growth in a dose-dependent manner | 24623736 |
| Hs683 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=128.9 μM | 24495907 | |
| U87 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=18.45 μM | 24495907 | |
| LNZ308 | Growth Inhibition Assay | 0-1000 μM | 96 h | IC50=326.7 μM | 24495907 | |
| U87 | Apoptosis Assay | 100 μM | 48 h | DMSO | increases the caspase-3/7 activity | 24481586 |
| U251 | Apoptosis Assay | 100 μM | 48 h | DMSO | increases the caspase-3/7 activity | 24481586 |
| U251 | Growth Inhibition Assay | 24 h | IC50=86.29 ± 1.58 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 48 h | IC50=75.34 ± 1.02 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 72 h | IC50=72.42 ± 1.45 μM | 24326954 | ||
| U251 | Growth Inhibition Assay | 96 h | IC50=69.82 ± 3.04 μM | 24326954 | ||
| T98G | Growth Inhibition Assay | 0-750 μM | 72/96 h | inhibits cell viability in a dose dependent manner | 24324080 | |
| U251-MG | Growth Inhibition Assay | 0-800 μM | 72 h | inhibits cell viability in a dose dependent manner | 24093630 | |
| D54-MG | Growth Inhibition Assay | 0-800 μM | 72 h | inhibits cell viability in a dose dependent manner | 24093630 | |
| SHG-44 | Growth Inhibition Assay | 10-200 μM | 96 h | IC50=9.73 ± 2.12 μM | 24065569 | |
| U373 | Growth Inhibition Assay | 10-200 μM | 96 h | IC50=10.13 ± 1.02 μM | 24065569 | |
| HT-29 | Function Assay | 500 μM | 24/48 h | enhances the levels of γ-H2AX | 24038068 | |
| PC-3 | Growth Inhibition Assay | 0-25 μM | 48 h | inhibits cell growth which can be potentiated by lycopene | 23746934 | |
| PC-3 | Apoptosis Assay | 25 μM | 48 h | induces apoptosis which can be potentiated by lycopene | 23746934 | |
| T98G | Growth Inhibition Assay | 50-400 μM | 144 h | inhibits cell viability in a dose dependent manner | 23715499 | |
| U87-MG | Growth Inhibition Assay | 100 µM | 72 h | inhibits cell growth which can be enhanced by GTB | 23696788 | |
| U251-MG | Growth Inhibition Assay | 100 µM | 72 h | inhibits cell growth which can be enhanced by GTB | 23696788 | |
| LNT-229 | Growth Inhibition Assay | 3-100 μM | 24 h | inhibits clonogenic survival in a dose-dependent manner | 23667632 | |
| T98G | Growth Inhibition Assay | 10-700 μM | 24 h | inhibits clonogenic survival in a dose-dependent manner | 23667632 | |
| U87 | Function Assay | 100 µM | 3 h | elevates the levels of pChk1 and pChk2 | 23667469 | |
| HCT116 | Function Assay | 100 µM | 3 h | induces the Chk1 Phosphorylation | 23667469 | |
| HCT3-6 | Function Assay | 100 µM | 3 h | induces the Chk1 Phosphorylation | 23667469 | |
| U-87 | Growth Inhibition Assay | 0-40 μM | 12 d | inhibits cell growth in a dose-dependent manner | 23645729 | |
| U-87 | Apoptosis Assay | 0-40 μM | 3/6 d | induces apoptosis in both dose- and time-dependent manner | 23645729 | |
| U-87 | Function Assay | 0-40 μM | 3/6 d | induces autophagy in both dose- and time-dependent manner | 23645729 | |
| GB-SCC010 | Growth Inhibition Assay | 4 d | IC50=226 μM | 23612755 | ||
| GB-SCC026 | Growth Inhibition Assay | 4 d | IC50=53.1 μM | 23612755 | ||
| GB-SCC028 | Growth Inhibition Assay | 4 d | IC50=167 μM | 23612755 | ||
| U87 | Growth Inhibition Assay | 4 d | IC50=45.2 μM | 23612755 | ||
| U87 stem cell | Growth Inhibition Assay | 4 d | IC50=66.7 μM | 23612755 | ||
| TLX5 lymphoma | Cytotoxicity assay | IC50 = 5 μM | 7739008 | |||
| GM892 A | Cytotoxicity assay | IC50 = 7.6 μM | 7739008 | |||
| TLX5 lymphoma | Cytotoxicity assay | IC50 = 5 μM | 12459014 | |||
| HCT116 | Cytotoxicity assay | 4 days | IC50 = 4.34 μM | 19800803 | ||
| SNB75 | Antiproliferative assay | 10 uM | 24 hrs | Antiproliferative activity against human SNB75 cells at 10 uM after 24 hrs by SRB assay | 22268526 | |
| C6 | Antiproliferative assay | 100 uM | 48 hrs | Antiproliferative activity against rat C6 cells at 100 uM after 48 hrs by neutral red incorporation assay | 22268526 | |
| SF295 | Antiproliferative assay | 10 uM | 24 hrs | Antiproliferative activity against human SF295 cells at 10 uM after 24 hrs by SRB assay | 22268526 | |
| U87 | Antiproliferative assay | 5 days | IC50 = 49 μM | 22608389 | ||
| U138MG | Cytotoxicity assay | 48 hrs | IC50 = 26 μM | 23069682 | ||
| C6 | Cytotoxicity assay | 48 hrs | IC50 = 34 μM | 23069682 | ||
| A2780 | Antitumor assay | 5 days | IC50 = 8.5 μM | 23895620 | ||
| A2058 | Antitumor assay | 5 days | IC50 = 35.5 μM | 23895620 | ||
| SNB19 | Antitumor assay | 5 days | IC50 = 37 μM | 23895620 | ||
| M8 | Apoptosis assay | 50 to 100 uM | 48 hrs | Induction of apoptosis in human M8 cells assessed as apoptotic/necrotic cells at 50 to 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis | 24125877 | |
| SK-MEL-30 | Apoptosis assay | 100 uM | 48 hrs | Induction of apoptosis in human SK-MEL-30 cells assessed as apoptotic/necrotic cells at 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| SK-MEL-30 | Apoptosis assay | 50 uM | 48 hrs | Induction of apoptosis in human SK-MEL-30 cells assessed as apoptotic/necrotic cells at 50 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| MNT1 | Apoptosis assay | 100 uM | 48 hrs | Induction of apoptosis in human MNT1 cells assessed as apoptotic/necrotic cells at 100 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| MNT1 | Apoptosis assay | 50 uM | 48 hrs | Induction of apoptosis in human MNT1 cells assessed as apoptotic/necrotic cells at 50 uM after 48 hrs by APC-labeled annexin V and 7-AAD staining-based flow cytometric analysis relative to control | 24125877 | |
| A2780 | Cytotoxicity assay | 5 days | Cytotoxicity against human A2780 cells after 5 days by MTT assay | 24900418 | ||
| A2780/CP70 | Cytotoxicity assay | 5 days | Cytotoxicity against MMR-deficient human A2780/CP70 cells after 5 days by MTT assay | 24900418 | ||
| GBM 047T | Antitumor assay | 20 uM | 1 to 2 weeks | Tumoricidal effect in patient derived GBM 047T cells assessed as reduction in neurosphere formation at 20 uM after 1 to 2 weeks by 3D tumor clonogenic assay | 26355532 | |
| GBM 464T | Antitumor assay | 20 uM | 1 to 2 weeks | Tumoricidal effect in patient derived GBM 464T cells assessed as reduction in neurosphere formation at 20 uM after 1 to 2 weeks by 3D tumor clonogenic assay | 26355532 | |
| U87MG | Function assay | 3 hrs | Induction of DNA alkylation in human U87MG cells assessed as increase in N7-MedG formation after 3 hrs by LC-MS/MS analysis | 27614414 | ||
| U87MG | Antitumor assay | Antitumor activity against human U87MG cells orthotopically xenografted in Harlan nude mouse brain assessed as induction of slow tumor growth at 50 umol/kg, iv administered once daily for 5 days | 27614414 | |||
| U87MG | Antitumor assay | Antitumor activity against human U87MG cells orthotopically xenografted in Harlan nude mouse brain assessed as increase in mouse survival at 50 umol/kg, iv administered once daily for 5 days | 27614414 | |||
| MDCK | Cytotoxicity assay | 24 hrs | Cytotoxicity against MDCK cells expressing carbonic anhydrase 9 assessed as reduction in cell viability incubated for 24 hrs measured after 7 days under normoxic condition by methylene blue staining based clonogenic survival assay | 27823879 | ||
| MDCK | Cytotoxicity assay | 24 hrs | Cytotoxicity against MDCK cells expressing carbonic anhydrase 9 assessed as reduction in cell viability incubated for 24 hrs measured after 7 days under hypoxic condition by methylene blue staining based clonogenic survival assay | 27823879 | ||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| C6 | Cytotoxicity assay | 4 days | EC50 = 16.5 μM | ChEMBL | ||
| U87 | Cytotoxicity assay | 72 hrs | IC50 = 19.38 μM | ChEMBL | ||
| SNB19 | Growth inhibition assay | 7 days | GI50 = 35.7 μM | ChEMBL | ||
| SNB19 | Growth inhibition assay | 7 days | GI50 = 45.6 μM | ChEMBL | ||
| U373 | Function assay | 100 uM | 72 hrs | Induction of double stranded DNA break in empty vector transfected human U373 cells assessed as increase in gamma-H2AX level at 100 uM after 72 hrs by flow cytometry | ChEMBL | |
| Glioma | Antitumor assay | Antitumor activity against Homo sapiens (human) Glioma cells xenografted in transgenic mouse assessed as mouse survival | ChEMBL | |||
| Klik om meer experimentele gegevens over cellijnen te bekijken | ||||||
Chemische informatie, opslag en stabiliteit
| Molecuulgewicht | 194.15 | Formule | C6H6N6O2 |
Opslag (vanaf de datum van ontvangst) | 3 years-20°C (in the dark)powder |
|---|---|---|---|---|---|
| CAS-nr. | 85622-93-1 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | NSC 362856,CCRG 81045,Methazolastone | Smiles | CN1C(=O)N2C=NC(=C2N=N1)C(=O)N | ||
Oplosbaarheid
|
In vitro |
DMSO
: 39 mg/mL
(200.87 mM)
Verwarmd met een waterbad van 50°C;
Geultrasoneerd;
Water : 10 mg/mL (超声加热五分钟) Ethanol : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo formulatiecalculator (heldere oplossing)
Stap 1: Voer onderstaande informatie in (Aanbevolen: een extra dier om rekening te houden met verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in de sectie oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-moedervloeistof: mg geneesmiddel vooropgelost in μL DMSO ( Concentratie moedervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de batch van het geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toeμL PEG300, mengen en verhelderen, daarna toevoegenμL Tween 80, mengen en verhelderen, daarna toevoegen μL ddH2O, mengen en verhelderen.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toe μL Maïsolie, mengen en verhelderen.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysieke methoden zoals vortexen, ultrasoon of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Kenmerken |
Methazolastone is a second-generation alkylating agent.
|
|---|---|
| Targets/IC50/Ki |
DNA replication
(L-1210, L-1210/BCNU cells) |
| In vitro |
Temozolomide (TMZ) veroorzaakt de vorming van alkalilabiele DNA-plaatsen die in vergelijkbare hoeveelheden aanwezig zijn en met een vergelijkbare snelheid worden hersteld in L-1210 en L-1210/BCNU cellijnen. In L-1210, maar niet in L-1210/BCNU, induceert deze verbinding een arrestatie van cellen in de SL-G2-M-fasen. De gevoeligheid van zowel chemo-gevoelige als resistente cellen (D54-R en U87-R) wordt significant verhoogd onder hyperoxie. Zowel het middel als hyperoxie zijn geassocieerd met verhoogde fosforylering van ERK p44/42 MAPK (Erk1/2), maar in mindere mate in D54-R cellen, wat suggereert dat Erk1/2-activiteit betrokken kan zijn bij de regulatie van hyperoxie en TMZ-gemedieerde celdood. Hyperoxie versterkt de toxiciteit in GBM-cellen door inductie van apoptosis, mogelijk via MAPK-gerelateerde routes. Het induceert in monocyten de DNA-schade responsroutes ATM-Chk2 en ATR-Chk1, wat resulteert in p53-activatie. Chronische blootstelling aan deze verbinding resulteert in verworven TMZ-resistentie en verhoogt de miR-21-expressie. Behandeling ermee triggert endoplasmatisch reticulum (ER) stress met verhoogde expressie van GADD153- en GRP78-eiwitten, en verlaagt pro-caspase 12-eiwit. Het induceert Autophagy via mitochondriale schade- en ER-stress-afhankelijke mechanismen om glioomcellen te beschermen. |
| In vivo |
Na een dagelijkse i.p. dosis van 40 mg/kg gedurende 5 opeenvolgende dagen (dagen 1-5 na tumortransplantatie), verhoogt TMZ (Temozolomide) de levensduur met 86% in L-1210 en 22% in L-1210/BCNU. In L-1210/BCNU wordt geen effect waargenomen na 100 μM of 200 μM behandeling; slechts 400 μM van deze verbinding produceerde een accumulatie van cellen in de premitotische fase, maar veel minder dan in L-1210. In L-1210/BCNU is de maximale accumulatie van cellen in SL-G2-M, na 48-72 uur, ongeveer 30% vergeleken met 23% in onbehandelde cellen. Cellen accumuleren in SL-G2-M traden ook op wanneer L-1210 leukemie-dragende muizen i.v. werden behandeld met het middel (40 mg/kg). Een dergelijk effect wordt niet waargenomen op L-1210/BCNU-cellen van muizen die dezelfde dosis van het medicijn kregen. |
Referenties |
|
Toepassingen
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
| Western blot | pERK / ERK / p-p38 / p38 DR5 / c-FLIP / Survivin / XIAP |
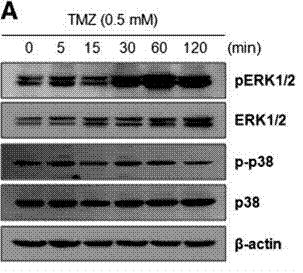
|
24436439 |
| Growth inhibition assay | Cell viability |
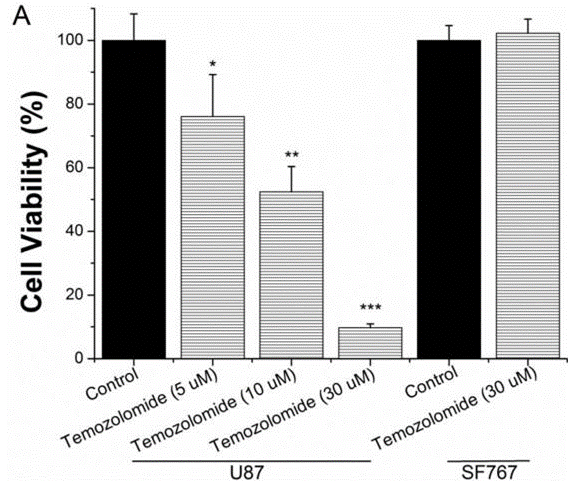
|
25751281 |
| Immunofluorescence | Phalloidin / Phospho-H2A.X cleaved caspase-3 |
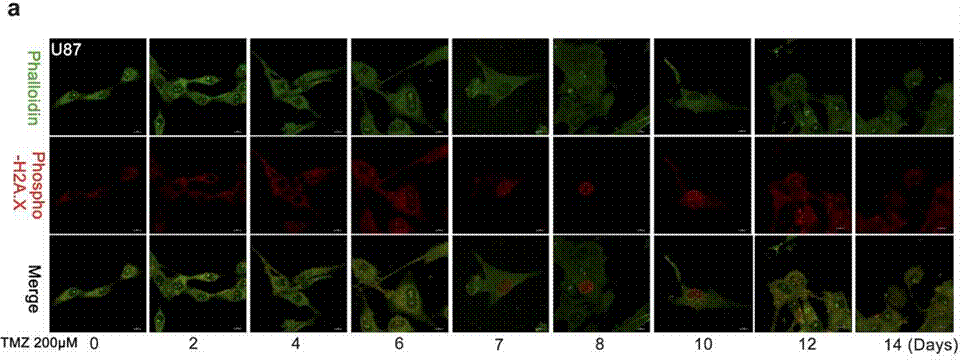
|
27375225 |
Informatie over klinische proeven
(gegevens van https://clinicaltrials.gov, bijgewerkt op 2024-05-22)
| NCT-nummer | Werving | Aandoeningen | Sponsor/medewerkers | Startdatum | Fasen |
|---|---|---|---|---|---|
| NCT05128734 | Not yet recruiting | Breast Cancer Triple Negative |
AHS Cancer Control Alberta |
July 1 2024 | Phase 2 |
| NCT06161974 | Not yet recruiting | High Grade Glioma|Astrocytoma|Astrocytoma Grade III|Astrocytoma Grade IV|Diffuse Intrinsic Pontine Glioma|WHO Grade III Glioma|WHO Grade IV Glioma|Metastatic Brain Tumor|Diffuse Midline Glioma H3 K27M-Mutant|Thalamus Tumor|Spinal Tumor|IDH1 Mutation|IDH1 R132|IDH1 R132C|IDH1 R132H|IDH1 R132S|IDH1 R132G|IDH1 R132L|Oligodendroglioma |
Rigel Pharmaceuticals|Nationwide Children''s Hospital |
June 2024 | Phase 2 |
| NCT04967690 | Not yet recruiting | Newly Diagnosed Glioblastoma |
Double Bond Pharmaceutical AB |
January 2024 | Phase 1 |
| NCT05698524 | Recruiting | Recurrent High Grade Glioma|Anaplastic Astrocytoma|Anaplastic Oligodendroglioma|Glioblastoma|Gliosarcoma |
University of Nebraska|Xynomic Pharmaceuticals Inc. |
June 26 2023 | Phase 1 |
| NCT04945148 | Not yet recruiting | Glioblastoma IDH-wildtype |
Hopital Foch|National Cancer Institute France |
May 2023 | Phase 2 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, laat dan een bericht achter.
