MEK is a member of kinases in MAPK signal pathway which can activates p38 MAPK and JNK when MEK is ACTIVATED by TNF-alpha, GPCR and so on. MEK could be regulated by MEKKs or RAF. The Raf / MEK / ERK signal transduction is involved in cell growth, cell proliferation and cell survival. [show the full text]
MEK remmers (MEK Inhibitors)
| Cat.nr. | Productnaam | Informatie | Citaties van productgebruik | Productvalidaties |
|---|---|---|---|---|
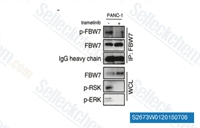
| S2673 | Trametinib (GSK1120212) | Trametinib (GSK1120212, JTP-74057) is een zeer specifieke en potente MEK1/2-remmer met een IC50 van 0,92 nM/1,8 nM in celvrije assays, en het remt de kinaseactiviteiten van c-Raf, B-Raf, ERK1/2 niet. Deze verbinding activeert autophagy en induceert apoptosis. |
|
|
| S1036 | PD0325901 (Mirdametinib) | Mirdametinib (PD0325901) is een selectieve en niet-ATP-competitieve MEK-remmer met een IC50 van 0,33 nM in celvrije assays, ruwweg 500-maal potenter dan CI-1040 op de fosforylering van ERK1 en ERK2. Fase 2. |
|

|
| S1008 | AZD6244 (Selumetinib) | Selumetinib (AZD6244, ARRY-142886) is een potente, zeer selectieve MEK-remmer met een IC50 van 14 nM voor MEK1 en een Kd-waarde van 530 nM voor MEK2. Het remt ook de ERK1/2-fosforylering met een IC50 van 10 nM, zonder remming van p38α, MKK6, EGFR, ErbB2, ERK2, B-Raf, enz. Selumetinib onderdrukt celproliferatie, migratie en veroorzaakt apoptosis. Fase 3. |
|

|
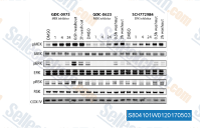
| S8041 | Cobimetinib (GDC-0973) | Cobimetinib (GDC-0973, RG7420) is een potente en zeer selectieve MEK1-remmer met een IC50 van 4,2 nM, en vertoont geen significante remming wanneer getest tegen een panel van meer dan 100 serine-threonine- en tyrosinekinasen. Deze verbinding induceert apoptosis. Fase 3. |
|
|
| S7170 | Avutometinib (Ro5126766, CH5126766) | Avutometinib(RO5126766,CH5126766,VS 6766, CKI-27, R-7304, RG-7304) is een dubbele RAF/MEK-remmer met een IC50 van respectievelijk 8,2 nM, 19 nM, 56 nM en 160 nM voor BRAF V600E, BRAF, CRAF en MEK1. Fase 1. |
|
|
| S1102 | U0126-EtOH | U0126-EtOH is een zeer selectieve remmer van MEK1/2 met een IC50 van 0,07 μM/0,06 μM in celvrije assays, 100 keer hogere affiniteit voor ΔN3-S218E/S222D MEK dan PD98059. U0126 remt autophagy en mitophagy met antivirale activiteit. |
|

|
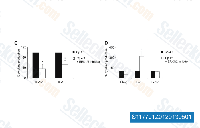
| S1177 | PD 98059 | PD98059 is een niet-ATP-competitieve MEK-remmer met een IC50 van 2 μM in een celvrije test, remt specifiek de MEK-1-gemedieerde activering van MAPK; remt ERK1 of ERK2 niet direct. PD98059 is een ligand voor de arylhydrocarbonreceptor (AHR) en functioneert als een AHR-antagonist. |
|
|
| S1020 | PD184352 (CI-1040) | PD184352 (CI-1040) is een ATP niet-competitieve MEK1/2 remmer met een IC50 van 17 nM in celgebaseerde assays, 100 keer selectiever voor MEK1/2 dan voor MEK5. Deze verbinding induceert selectief apoptosis. |
|

|
| S7007 | MEK162 (Binimetinib, ARRY-162) | Binimetinib (MEK162, ARRY-162, ARRY-438162) is een krachtige remmer van MEK1/2 met een IC50 van 12 nM in een celvrije test. Binimetinib induceert G1-celcyclusarrest en apoptose in menselijke NSCLC-cellijnen en induceert autophagy. Fase 3. |
|

|
| S1531 | BIX 02189 | BIX02189 is een selectieve remmer van MEK5 met een IC50 van 1,5 nM, remt ook de katalytische activiteit van ERK5 met een IC50 van 59 nM in celvrije assays, en remt geen nauw verwante kinasen MEK1, MEK2, ERK2 en JNK2. |
|

|
In the mitogen-activated protein kinase (MAPK) pathway, receptor tyrosine kinase activation results in adaptor proteins phosphorylating RAS. This results in the activation of the RAF-MEK-ERK kinase signalling pathway, and consequently leads to the activation of several downstream substrates that affect a number of transcription factors. The knock-on effect is that a myriad of cellular processes such as cell proliferation, survival, transformation, translational control and cytoskeletal rearrangement. In oncology, the MAPK pathway is a key contributor to tumor progression, angiogenesis, and metastasis.
In the RAS-RAF-MEK-ERK pathway, MEK has been the target of oncology research. The MEK kinase is expressed from MEK1 and MEK2 – two genes that share ~80% structural homology – that display slightly different isoforms of MEK to produce potentially different functions. Both MEK1 and MEK2 kinases are implicated in ~30% of all human cancers where MAPK signalling pathway is involved.[1] These dual-specificity kinases phosphorylate both tyrosine and threonine residues; MEK1 and MEK2 sequentially phosphorylate ERK1 at 185Tyr and then at 183Thr. MEK exists just downstream of RAF in the classical MAPK pathway known as RAS-RAF-MEK-ERK pathway. Phosphorylation of MEK by RAF results in the phosphorylation of ERK1 and ERK2. MEK kinases show very high specificity for ERK, in fact it is the only known substrate for MEK. Therefore, constitutive phosphorylation of MEK in the RAF-MEK-ERK kinase pathway occurs by either the overexpression or mutation of receptor tyrosine kinases, and/or mutations of RAS and RAF (A-RAF and B-RAF).[2]
The MEK enzyme itself consists of hydrophobic allosteric pockets adjacent to the ATP-binding site that facilitates the design of highly selective allosteric inhibitors. This is in contRASt to the many kinases for which there is no allosteric-binding site. Consequently, this feature is recognized by many pharmaceutical companies as a characteristic that facilitates more selective inhibitor design since the more conserved ATP-binding site is not directly targeted. MEK1 and MEK2 are positioned at the focal point of many mitogenic signaling pathways that integrates into the ERK pathway. Characteristics such as unusually restricted and unique substrate specificities, plus the integrating role of mitogenic signaling pathways demonstrates the benefits of developing a MEK inhibitor against the ERK pathway.[3]
The utility of targeting MEK inhibition is likely to be best realized among tumors where the MEK pathway is constitutively activated. Such a scenario includes activating mutations of BRAF that results in tumors that are dependent to MEK signaling, and consequently very sensitive to MEK inhibition.[4] This is likely to be the case among a sub-population of BRAF mutations observed in melanoma and thyroid cancers. Currently, MEK inhibition is likely to prove most effective when used in a combination strategy. This is because there is cross-talk involved between RAS-RAF-MEK-ERK and the PI3K-AKT pathway. As a consequence, inhibition of one pathway leads to constitutive signalling in the other. This is a reflection of the complexity of the kinase signalling pathways implicated in cancer.[2]
Aside from anti-tumor potential, MEK inhibition may play a role where inflammation is concerned. Several key protein downstream of MEK are involved in inflammatory responses including TNF, IL-1, and other cytokines. MEK signaling directly impacts both the expression of cytokines and subsequent activation pathways. Therefore, MEK inhibitors –particularly orally bioavailable compounds – may be suitable agents for the treatment of inflammatory disease. In addition, it should be noted that anaphylatoxins utilize the MEK kinase cascade to initiate disease processes such as arthritis.[2]
