alleen voor onderzoeksdoeleinden
AG-490 EGFR remmer
Cat.nr.S1143
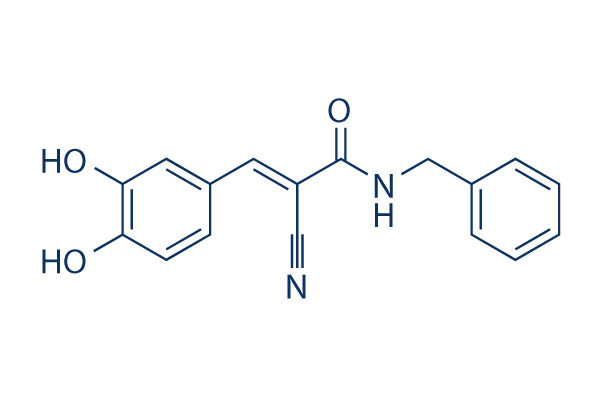
Chemische structuur
Molecuulgewicht: 294.30
Kwaliteitscontrole
| Gerelateerde doelwitten | VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Overig EGFR Inhibitoren | Lazertinib (YH25448) Icotinib Hydrochloride Sunvozertinib AG-1478 Canertinib (CI-1033) WZ4002 Rociletinib (CO-1686) Poziotinib (NOV120101, HM781-36B) Genistein Allitinib tosylate |
Celkweek, behandeling & werkzame concentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| BC3 | Function Assay | 100 µM | 24 h | mediates PEL cell apoptosis | ||
| BCBL1 | Function Assay | 100 µM | 24 h | mediates PEL cell apoptosis | ||
| BC3 | Function Assay | 100 µM | 24 h | mediates de-phosphorylation of STAT3 correlated with HSP70 and HSF1 reduction | ||
| BCBL1 | Function Assay | 100 µM | 24 h | mediates de-phosphorylation of STAT3 correlated with HSP70 and HSF2 reduction | ||
| BC3 | Function Assay | 100 µM | 24 h | induces a complete autophagic flux | ||
| BCBL1 | Function Assay | 100 µM | 24 h | induces a complete autophagic flux | ||
| SK-MEL-28 | Function Assay | 50/100 µM | 48 h | DMSO | reduces anoikis resistance | |
| MeWo | Function Assay | 50/100 µM | 48 h | DMSO | reduces anoikis resistance | |
| SK-MEL-5 | Function Assay | 50/100 µM | 48 h | DMSO | reduces anoikis resistance | |
| SK-MEL-2 | Function Assay | 50/100 µM | 48 h | DMSO | reduces anoikis resistance | |
| B16-F0 | Function Assay | 50/100 µM | 48 h | DMSO | reduces anoikis resistance | |
| TRPM2/HEK | Function Assay | 0.1–25 µM | 15 min | DMSO | reduces H2O2-induced Ca2+increase in a concentration-dependent manner, and the IC50 value was 1.7 µM | |
| U937 | Function Assay | 0.1–25 µM | 15 min | DMSO | reduces H2O2-induced Ca2+increase in a concentration-dependent manner, and the IC50 value was 0.4 µM | |
| TRPM2/HEK | Function Assay | 10 µM | 40 min | DMSO | reduces TRPM2 activation even at high concentrations of H2O2 | |
| GL37 | Cell Viability Assay | 0-10 µM | 48 h | suppresses La expression | ||
| NRK-52E | Function Assay | 1 µM | 10 min | blocks the stimulatory effect of Ang II on Pax-2 expression | ||
| NRK-52E | Function Assay | 1 µM | 10 min | blocks Ang II induced CD24 expression | ||
| HSC | Function Assay | 20 μM | 1 h | abrogates the differential effects of leptin or AGEs | ||
| EJ | Growth Inhibition Assay | 50/80 μM | 24/48/72 h | inhibits cell growth in both time and dose dependent manner | ||
| EJ | Growth Inhibition Assay | 50/80 μM | 48 h | causes S-phase arrest | ||
| EJ | Function Assay | 50/80 μM | 48 h | downregulates c-Myc, cyclinD1, survivin and VEGF expressions | ||
| HepG2 | Function Assay | 50-500 μM | 60 min | inhibits the IL-6-induced phosphorylation of STAT1 (Tyr705) and STAT3 (Tyr705) in a dose-dependent manner | ||
| SGC7901 | Cell Viability Assay | 0-100 μM | 24/48/72 h | causes a significant reduction in cell viability dose-dependently but not time-dependently | ||
| AGS | Cell Viability Assay | 0-100 μM | 24/48/72 h | causes a significant reduction in cell viability dose-dependently but not time-dependently | ||
| SGC7901 | Function Assay | 50 μM | 24/48/72 h | the levels of pJAK2 began to decline at 24 hr, and rebounded at 72 hr | ||
| AGS | Function Assay | 50 μM | 24/48/72 h | the levels of pJAK2 began to decline at 24 hr, and rebounded at 72 hr | ||
| SGC7901 | Function Assay | 50 μM | 24/48/72 h | the cytoplasmic localization of pJAK2 (JAK2 phosphorylated at residues Tyr1007 and Tyr1008) decreased after AG490 treatment for 24 and 48 hr, but started to rebound at 72 hr | ||
| AGS | Function Assay | 50 μM | 24/48/72 h | the cytoplasmic localization of pJAK2 (JAK2 phosphorylated at residues Tyr1007 and Tyr1008) decreased after AG490 treatment for 24 and 48 hr, but started to rebound at 72 hr | ||
| MC3T3-E1 | Function Assay | 50 μM | 4 h | inhibits HSE-induced BMP7 and GHR protein expression | ||
| 7TD1-DXM | Growth Inhibition Assay | 10 μM | 72 h | DMSO | inhibits cell growth | |
| 7TD1-WD-90 | Growth Inhibition Assay | 10 μM | 72 h | DMSO | inhibits cell growth | |
| 7TD1-DXM | Apoptosis Assay | 50 μM | 48 h | DMSO | induces apoptosis | |
| 7TD1-WD-90 | Apoptosis Assay | 50 μM | 48 h | DMSO | induces apoptosis | |
| 7TD1-WD-90 | Function Assay | 50 μM | 6 h | DMSO | significantly inhibits the phosphorylation of JAK2 and phosphorylation of STAT3 | |
| HepG2 | Function Assay | 100 μM | 12/24 h | inhibits STAT3 tyrosine phosphorylation | ||
| RAW264.7 | Function Assay | 50 μM | 24/48 h | suppresses RANKL-induced osteoclastogenesis | ||
| RAW264.7 | Growth Inhibition Assay | 0-50 μM | 48 h | inhibits cell growth dose-dependently | ||
| RAW264.7 | Growth Inhibition Assay | 0-50 μM | 48 h | causes an arrest of RAW264.7 cells at the G0/G1 phase of the cell cycle | ||
| RAW264.7 | Function Assay | 50 μM | 24/48 h | inhibits RANKL-induced NFATc1 expression and phosphorylation of Ser727STAT3 | ||
| A549 | Function Assay | 20/40 μM | 20 h | 20 μM AG490 suppresses the radiation-induced invasion of A549 cells | ||
| A549 | Function Assay | 10/20/40 μM | 24 h | suppresses the radiation-induced elevation of VEGF | ||
| HUVECs | Cell Viability Assay | 20 µM | 4 h | attenuates H2O2-induced cell shrinkage and improved the attachment rate of the cells | ||
| HUVECs | Apoptosis Assay | 20 µM | 4 h | significantly decreases the cell apoptotic index | ||
| BV-2 | Function Assay | 20 µM | 16 h | inhibits LPS-induced STAT1 phosphorylation with almost completely diminished iNOS expression | ||
| NRK-52E | Function Assay | 5 μM | 30 min | attenuates Ang-(1–7)-inhibited TGF-β1 mRNA at 16 h | ||
| SW620 | Function Assay | 20 µM | 1/6 h | inhibits p-STAT3 activation | ||
| RPE | Function Assay | 30 µM | 3 h | inhibits the induction of p-STAT3 expression | ||
| SW1116 | Function Assay | 100 µM | 24/48/72 h | decreases the expression of JAK2 and pJAK2 time-dependently | ||
| HT29 | Function Assay | 100 µM | 24/48/72 h | decreases the expression of JAK2 and pJAK2 time-dependently | ||
| SW1116 | Function Assay | 100 µM | 24/48/72 h | decreases the pSTAT3 levels in a time-dependent manner | ||
| HT29 | Function Assay | 100 µM | 24/48/72 h | decreases the pSTAT3 levels in a time-dependent manner | ||
| ARPE-19 | Function Assay | 5 μM | 30 min | inhibits JAK2 phosphorilation | ||
| HSC-T6 | Apoptosis Assay | 10 μM | 2 h | inhibits the apoptosis of HSC-T6 cells induced by CDE | ||
| HSC-T6 | Function Assay | 10 μM | 2 h | inhibits the expressions of pY-STAT1 and Bad induced by CDE | ||
| Hep-2 | Growth Inhibition Assay | 25-100 μM | 24/48/72 h | inhibits cell growth in both time and dose dependent manner | ||
| Hep-2 | Apoptosis Assay | 50 μM | 24/48/72 h | induces cell apoptosis time dependently | ||
| Hep-2 | Function Assay | 50 μM | 24/48/72 h | inhibits G1 to S cell cycle transition and induces G1 cell cycle arrest | ||
| Hep-2 | Function Assay | 50 μM | 24/48/72 h | downregulates the STAT3, p-STAT3 and survivin protein levels | ||
| KF8 | Function Assay | 10 μM | 1 h | DMSO | inhibits IL-33-induced NF-κB activation | |
| KF8 | Function Assay | 10 μM | 1 h | DMSO | inhibits IL-33-induced IκBα degradation and NF-κB activation | |
| HEL | Function Assay | 100 μM | 12-72 h | inhibits the level of p-JAK2, JAK2 | ||
| HEL | Growth Inhibition Assay | 100 μM | 0-5 d | reduces growth of JAK2V617F-expressing HEL cells | ||
| A-172 | Function Assay | 50/100 μM | 48 h | reduces the levels of constitutively activated STAT3 in a time-dependent and dose-dependent fashion | ||
| MZ-18 | Function Assay | 50/100 μM | 48 h | reduces the levels of constitutively activated STAT3 in a time-dependent and dose-dependent fashion | ||
| MZ-54 | Function Assay | 50/100 μM | 48 h | reduces the levels of constitutively activated STAT3 in a time-dependent and dose-dependent fashion | ||
| MZ-256 | Function Assay | 50/100 μM | 48 h | reduces the levels of constitutively activated STAT3 in a time-dependent and dose-dependent fashion | ||
| MZ-304 | Function Assay | 50/100 μM | 48 h | reduces the levels of constitutively activated STAT3 in a time-dependent and dose-dependent fashion | ||
| A-172 | Growth Inhibition Assay | 50/100 μM | 48 h | leads to a statistically significant reduction of cell proliferation over a time period of 48 h | ||
| MZ-18 | Growth Inhibition Assay | 50/100 μM | 48 h | leads to a statistically significant reduction of cell proliferation over a time period of 48 h | ||
| MZ-54 | Growth Inhibition Assay | 50/100 μM | 48 h | leads to a statistically significant reduction of cell proliferation over a time period of 48 h | ||
| MZ-256 | Growth Inhibition Assay | 50/100 μM | 48 h | leads to a statistically significant reduction of cell proliferation over a time period of 48 h | ||
| MZ-304 | Growth Inhibition Assay | 50/100 μM | 48 h | leads to a statistically significant reduction of cell proliferation over a time period of 48 h | ||
| A-172 | Function Assay | 50/100 μM | 48 h | inhibits migration | ||
| MZ-18 | Function Assay | 50/100 μM | 48 h | inhibits migration | ||
| MZ-54 | Function Assay | 50/100 μM | 48 h | inhibits migration | ||
| MZ-256 | Function Assay | 50/100 μM | 48 h | inhibits migration | ||
| MZ-304 | Function Assay | 50/100 μM | 48 h | inhibits migration | ||
| A-172 | Function Assay | 100 μM | 48 h | inhibits invasion | ||
| MZ-18 | Function Assay | 100 μM | 48 h | inhibits invasion | ||
| MZ-54 | Function Assay | 100 μM | 48 h | inhibits invasion | ||
| MZ-256 | Function Assay | 100 μM | 48 h | inhibits invasion | ||
| MZ-304 | Function Assay | 100 μM | 48 h | inhibits invasion | ||
| A-172 | Function Assay | 50/100 μM | 48 h | reduces transcription of MMP genes and reduces enzymatic activity of MMPs | ||
| MZ-18 | Function Assay | 50/100 μM | 48 h | reduces transcription of MMP genes and reduces enzymatic activity of MMPs | ||
| MZ-54 | Function Assay | 50/100 μM | 48 h | reduces transcription of MMP genes and reduces enzymatic activity of MMPs | ||
| MZ-256 | Function Assay | 50/100 μM | 48 h | reduces transcription of MMP genes and reduces enzymatic activity of MMPs | ||
| MZ-304 | Function Assay | 50/100 μM | 48 h | reduces transcription of MMP genes and reduces enzymatic activity of MMPs | ||
| SW1990 | Growth Inhibition Assay | 20 μM | 24/48/72 h | inhibits cell growth time dependently | ||
| SW1990 | Function Assay | 20 μM | 24 h | decreases the expression of MMP-2 and VEGF mRNAs | ||
| SW1990 | Function Assay | 20 μM | 24 h | decreases the intensity of p-Stat3 expression | ||
| SW1990 | Invasion Assay | 20 μM | 24 h | reduces invasion of SW1990 cells | ||
| THP1 | Function Assay | 10 uM | 30 min | inhibits STAT3 tyrosine phosphorylation by over 60% | ||
| BMMC | Function Assay | 0-10 μM | 15 min | inhibits LTC4 release in a dose-dependent fashion with near complete inhibition at concentrations ⩾10 μM | ||
| A549 | Function Assay | 15 μm | 1 h | inhibits the phosphorylation of STAT1 on tyrosine 701 was detected 15 min after SPE B treatment | ||
| OVCAR-3 | Function Assay | 10 uM | 1 h | inhibits LPA-induced STAT3 phosphorylation | ||
| PA-1 | Function Assay | 10 uM | 1 h | inhibits LPA-induced STAT3 phosphorylation | ||
| OVCAR-3 | Function Assay | 10 uM | 1 h | inhibits LPA-induced ovarian cancer cell motility | ||
| PA-1 | Function Assay | 10 uM | 1 h | inhibits LPA-induced ovarian cancer cell motility | ||
| Jurkat | Growth Inhibition Assay | 50 μM | 24/48/72 h | enhances TRAIL-induces cell growth inhibition | ||
| SUPT1 | Growth Inhibition Assay | 50 μM | 24/48/72 h | enhances TRAIL-induces cell growth inhibition | ||
| Jurkat | Apoptosis Assay | 50 μM | 24/48 h | enhances TRAIL-induces cell apoptosis | ||
| SUPT1 | Apoptosis Assay | 50 μM | 24/48 h | enhances TRAIL-induces cell apoptosis | ||
| Klik om meer experimentele gegevens over cellijnen te bekijken | ||||||
Chemische informatie, opslag en stabiliteit
| Molecuulgewicht | 294.30 | Formule | C17H14N2O3 |
Opslag (vanaf de datum van ontvangst) | |
|---|---|---|---|---|---|
| CAS-nr. | 133550-30-8 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | Tyrphostin B42, Zinc02557947 | Smiles | C1=CC=C(C=C1)CNC(=O)C(=CC2=CC(=C(C=C2)O)O)C#N | ||
Oplosbaarheid
|
In vitro |
DMSO
: 58 mg/mL
(197.07 mM)
Ethanol : 9 mg/mL Water : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo formulatiecalculator (heldere oplossing)
Stap 1: Voer onderstaande informatie in (Aanbevolen: een extra dier om rekening te houden met verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in de sectie oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-moedervloeistof: mg geneesmiddel vooropgelost in μL DMSO ( Concentratie moedervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de batch van het geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toeμL PEG300, mengen en verhelderen, daarna toevoegenμL Tween 80, mengen en verhelderen, daarna toevoegen μL ddH2O, mengen en verhelderen.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toe μL Maïsolie, mengen en verhelderen.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysieke methoden zoals vortexen, ultrasoon of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
JAK2 (V617F)
EGFR
(Cell-free assay) 0.1 μM
|
|---|---|
| In vitro |
AG-490 remt HER-2-gedreven celproliferatie met een IC50 van 3,5 μM. Overeenkomend met de specifieke dosisafhankelijke remming van constitutief geactiveerde JAK2 in pre-B acute leukemie (ALL) cellen, blokkeert deze verbinding (5 μM) bijna volledig de groei van alle ALL-cellen door geprogrammeerde celdood te induceren, zonder nadelig effect op normale hematopoëse. Deze chemische stof remt de activiteiten van Lck, Lyn, Btk, Syk en Src niet. Het (60-100 μM) blokkeert de constitutieve activering van Stat3sm en remt zowel de spontane als de interleukine 2-geïnduceerde groei van mycosis fungoides (MF) tumorcellen met respectievelijk IC50-waarden van 75 μM en 20 μM. Deze verbinding remt krachtig IL-2-gemedieerde humane T-celgroei met een IC50 van 25 μM door de activiteiten van JAK3 en STAT5a/b te blokkeren. Het remt significant de constitutieve activering van Stat3 in MOPC-, MPC11- en S194-cellen, wat leidt tot dramatische dosisafhankelijke apoptose. Deze chemische stof (100 μM) remt Akt-fosforylering, remt de activering van nucleaire factor-κB en veroorzaakt de activering van GSK-3β, wat leidt tot de vermindering van c-Myc. Het (50 μM) kan apoptose induceren van BaF3-cellen die T315I- en E255K-mutanten van Bcr-Abl tot expressie brengen. Deze verbinding bij 30 μM remt niet alleen Epo-geïnduceerde fosforylering van wild-type JAK2, maar ook constitutieve fosforylering van de JAK2 V617F-mutant. Het remt ook krachtig cytokine-onafhankelijke celgroei geïnduceerd door de JAK2 V617F-mutant in BaF3-cellen. |
| Kinase Assay |
In vitro kinase autofosforylatie
|
|
AG-490 wordt opgelost in DMSO 10%-H2O-ethanol 45%. Ruwe membraanextracten (0,125 μg/mL) worden gedurende 15 minuten bij 4 °C voorgeactiveerd met EGF (20 nM) in 50 mM HEPES-buffer, pH 7,6, en 125 mM NaCl. Autofosforylatieactiviteit van EGFR- of ErbB2-kinase wordt gedurende 30 seconden bij 4 °C in V-vormige 96-wells platen getest. Membraanextracten (8 μL) worden aan elke well toegevoegd die reactiemengsel (12 μL, 50 mM HEPES, pH 7,4, 125 mM NaCl, 12 mM M8Ac2, 2 mM MnCl2, 1 mM NaVO3, 1 μM ATP en 1 μCi [γ-32P]ATP, eindconcentraties) en toenemende concentraties van deze verbinding (4 μL) bevat. Na beëindiging door toevoeging van hete monsterbuffer worden de monsters op een 6% SDS-polyacrylamidegelelektroforese-minigel geladen, de gels gedroogd en autoradieografie uitgevoerd gedurende de lineaire belichtingstijd. De receptorbanden worden densitometrisch gescand en de resultaten geanalyseerd met het Ez-Fit-programma. Voor de analyse van autofosforylatie van JAK2 wordt JAK2 geïmmunoprecipiteerd met behulp van anti-JAK2-antilichaam uit lysaten van G2-cellen die gedurende 16 uur zijn voorbehandeld met toenemende concentraties van deze chemische stof (0-50 μM). Immuuncomplexen worden vervolgens geïmmunoblot met anti-fosfotyrosine-antilichaam. Een dosisafhankelijke remming van in vitro kinase-activiteit wordt aangetoond door de autofosforylatie van JAK2 te beoordelen.
|
|
| In vivo |
Toediening van AG-490 vermindert drastisch het aantal CD45+ en HLA-DR+ cellen van 48% en 46% in het beenmerg van onbehandelde muizen, evenals 38% en 22% in de milt van onbehandelde muizen tot ondetecteerbare niveaus. In vivo toediening van deze verbinding veroorzaakt apoptose van murine myeloom tumorcellen, maar remt IL-12-gemedieerde macrofaagactivering en IFN-γ-productie door lymfocyten niet. In overeenstemming met de in vitro blokkering van JAK2 V617F-mutante activiteit, remt behandeling met deze chemische stof van 0,5 mg/dag gedurende 10 dagen effectief JAK2 V617F-mutant-geïnduceerde tumorigenese en tumorcelinvasie in naakte muizen. Gecombineerde therapie met dit middel en IL-12 induceert grotere antitumorale effecten dan elk middel afzonderlijk in een murien myeloom tumormodel. |
Referenties |
|
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, laat dan een bericht achter.
Veelgestelde vragen
Vraag 1:
I would like to know whether it (S1143) goes to CNS through BBB, or not?
Antwoord:
It can go through the BBB, as shown in this reference: http://bloodjournal.hematologylibrary.org/content/111/4/2062.full.html.
