Vascular Endothelial Growth Factor Receptor (VEGFR) is the receptor of VEGF. VEGFR is involved in cell proliferation, migration, survival and permeability. The VEGFs include five known structurally-related mammalian ligands (VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor, PLGF) and there are also three structurally related VEGFRs subtypes (VEGFR1, VEGFR2, and VEGFR3). [show the full text]
VEGFR remmers (VEGFR Inhibitors)
Overige Protein Tyrosine Kinase Inhibitoren
| Cat.nr. | Productnaam | Informatie | Citaties van productgebruik | Productvalidaties |
|---|---|---|---|---|
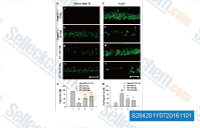
| S2842 | SAR131675 | SAR131675 is een VEGFR3-remmer met een IC50/Ki van 23 nM/12 nM in celvrije assays, ongeveer 50- en 10-maal selectiever voor VEGFR3 dan VEGFR1/2, weinig activiteit tegen Akt1, CDKs, PLK1, EGFR, IGF-1R, c-Met, Flt2 etc. |
|
|
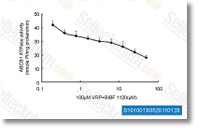
| S1010 | BIBF 1120 (Nintedanib) | Nintedanib is een krachtige drievoudige angiokinaseremmer voor VEGFR1/2/3, FGFR1/2/3 en PDGFRα/β met IC50 van 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM en 59 nM/65 nM in celvrije assays. Fase 3. |
|
|
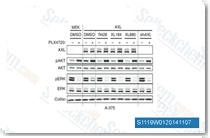
| S1119 | Cabozantinib (XL184) | Een potente VEGFR2-remmer met een IC50 van 0,035 nM, Cabozantinib (XL184) remt ook c-Met, Ret, Kit, Flt-1/3/4, Tie2 en AXL met een IC50 van respectievelijk 1,3 nM, 4 nM, 4,6 nM, 12 nM/11,3 nM/6 nM, 14,3 nM en 7 nM in celvrije assays. Het induceert PUMA-afhankelijke apoptosis in darmkankercellen via de AKT/GSK-3β/NF-κB-signaalroute. |
|
|
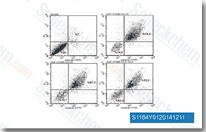
| S1164 | E7080 (Lenvatinib) | Lenvatinib is een multi-target remmer, voornamelijk voor VEGFR2(KDR)/VEGFR3(Flt-4) met IC50 van 4 nM/5,2 nM, minder potent tegen VEGFR1/Flt-1, ~10 keer selectiever voor VEGFR2/3 tegen FGFR1, PDGFRα/β in celvrije assays. Lenvatinib (E7080) remt ook FGFR1-4, PDGFR, Kit (c-Kit), RET (c-RET), en vertoont krachtige antitumoractiviteiten. Fase 3. |
|
|
| S1005 | Axitinib (AG-013736) | Axitinib is een multi-target remmer van VEGFR1, VEGFR2, VEGFR3, PDGFRβ en c-Kit met IC50 waarden van respectievelijk 0,1 nM, 0,2 nM, 0,1-0,3 nM, 1,6 nM en 1,7 nM in endotheelcellen van de varkensaorta. |
|
|
| S7667 | SU 5402 | SU5402 is een krachtige multi-getargete receptor tyrosinekinase-remmer met een IC50 van respectievelijk 20 nM, 30 nM en 510 nM voor VEGFR2, FGFR1 en PDGF-Rβ. |
|

|
| S8401 | Erdafitinib (JNJ-42756493) | Erdafitinib is een potente en selectieve oraal biologisch beschikbare, pan-fibroblast growth factor receptor (FGFR) remmer met potentiële antineoplastische activiteit. Deze verbinding bindt ook aan RET (c-RET), CSF-1R, PDGFR-α/PDGFR-β, FLT4, Kit (c-Kit) en VEGFR-2 en induceert cellulaire apoptose. |
|

|
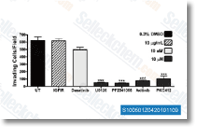
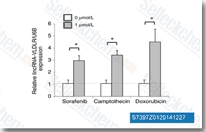
| S7397 | Sorafenib (BAY 43-9006) | Sorafenib is een multikinase-remmer van Raf-1 en B-Raf met respectievelijk een IC50 van 6 nM en 22 nM in celvrije assays. Sorafenib remt VEGFR-2, VEGFR-3, PDGFR-β, Flt-3 en c-KIT met een IC50 van respectievelijk 90 nM, 20 nM, 57 nM, 59 nM en 68 nM. Sorafenib induceert autophagy en apoptosis en activeert ferroptosis met antitumoractiviteit. |
|
|
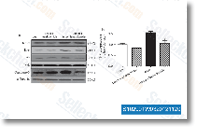
| S1029 | CC-5013 (Lenalidomide) | Lenalidomide is een TNF-α secretie-remmer met een IC50 van 13 nM in PBMC's. Lenalidomide (CC-5013) is een ligand van ubiquitin E3 ligase cereblon (CRBN) en veroorzaakt selectieve ubiquitinatie en afbraak van twee lymfoïde transcriptiefactoren, IKZF1 en IKZF3, door de CRBN-CRL4 ubiquitine ligase. Lenalidomide bevordert de expressie van cleaved caspase-3 en remt de expressie van VEGF en induceert apoptosis. |
|
|
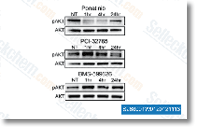
| S1490 | Ponatinib (AP24534) | Ponatinib is een nieuwe, krachtige multi-target remmer van Abl, PDGFRα, VEGFR2, FGFR1 en Src met IC50 van respectievelijk 0,37 nM, 1,1 nM, 1,5 nM, 2,2 nM en 5,4 nM in celvrije assays. Ponatinib (AP24534) remt autophagy. |
|
|
Solid tumors require the growth and dissemination of blood vessels and lymphatic vessels to support the metastatic growth of cancers. Following the recognition of growth factor receptor pathways that regulate angiogenesis, a number of small molecular inhibitors and antibodies have been developed that target the activity of vascular endothelial growth factor (VEGF)-VEGF receptor (VEGFR) pathway. This includes oral small-molecule tyrosine kinase inhibitors currently in clinical practice, namely sunitinib and sorafenib. These are commonly used in the treatment algorithm for renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC), two indications that are known to develop resistance to conventional chemotherapeutics.
The VEGFs include five known structurally-related mammalian ligands (VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor, PLGF). The VEGFs are disulfide-bonded homodimers, however, VEGFA and PLGF heterodimers are also known to exist. Due to alternative splicing or due to processing, VEGF ligands occur as several different variants. As a result, these variants bind differently to both VEGFRs and to co-receptors resulting in different biological responses including angiogenesis, lymphangiogenesis, permeability, inflammatory cell recruitment and fatty acid uptake. VEGFs are produced by several different cell types and act in a paracrine manner. The VEGFs bind to three structurally related tyrosine kinases (VEGFR1, VEGFR2, and VEGFR3). Modulating the effect of the VEGFRs are a number of co-receptors that lack intrinsic catalytic activity (i.e. heparin sulfate, neurophilins and integrins) and bind to VEGF.[1]
VEGFR1 (also known as Fms-like tyrosine kinase 1, Flt1, in mice) is a single-transmembrane glycoprotein structurally related to VEGFR2 and VEGFR3. VEGFR1 is expressed at high levels in vascular endothelial cells, and along with VEGFR2 binds to VEGFA. VEGFR1 is noted to bind exclusively to VEGFB and PIGF. Expression of VEGFR1 is noted to occur during vessel growth and remodeling activity. Non-endothelial cells that express VEGFR1 includes monocytes and macrophages, human tropholblasts, renal mesangial cells, vascular smooth muscle cells, dendritic cells and various tumor cells. A key regulator of VEGFR1 gene expression is hypoxia.[1]
VEGFR2 (also known as KDR; kinase insert domain receptor, in the human and Flk1; fetal liver kinase-1, in mice) binds VEGFA with a 10-fold lower affinity than VEGFR1. Other targets of VEGFR2 include proteolytically processed VEGFC and VEGFD. The only known ligand to uniquely bind to VEGFR2 is the open reading frame-encoded VEGFE. VEGFR2 is expressed in most adult vascular endothelial cells as well as circulating endothelial progenitor cells, pancreatic duct cells, retinal progenitor cells, megakaryocytes and hematopoietic cells. VEGFR2 expression is induced in conjunction with active angiogenesis (i.e. the uterus during the reproductive cycle) and in pathological process related to neovascularization (i.e. cancer). VEGFR2, often in combination with VEGFR3, is expressed at significantly upregulated levels in the tumor vascular endothelium in most common human solid tumors. Tumor cells can also express VEGFR2, however, epithelial and mesenchymal tumor cells typically express VEGFR1 rather than VEGFR2. Nevertheless, increased expression of VEGFR2 on tumor cells has been noted for melanoma and hematological malignancies. And, there is evidence supporting a relationship between chronic inflammation and tumor development.[1]
VEGFR3 (also known as Fms-like tyrosine kinase 4, Flt4 in the mouse) is activated by the binding of VEGFC or VEGFD, once these two ligands undergo proteolytic processing (this increases their affinity to VEGFR2 and VEGFR3). In addition, hVEGFD shows similar affinity to both VEGFR2 and VEGFR3, while mVEGFD binds only to VEGFR3. During embryogenesis, VEGFR3 expression occurs in the primary vascular plexus at day E8.5. In late stages of embryogenesis, VEGFR3 is expressed in venous endothelial cells of the cardinal vein, that results in VEGFR3-expressing lymphatics. Postnatally, VEGFR3 plays an important role in lymphatic endothelial cells, but its expression is also observed in endothelial cells engaged in active angiogenesis, such as tumor vessels, in endothelial tip cells of angiogenic sprouts in the developing retina or in chronic inflammatory wounds. The receptor is also found in non-endothelial cells such as osteoblasts, neuronal progenitors and macrophages – all of which may indirectly support angiogenesis. It remains unclear if tumor cells express VEGFR3. Despite this lack of clarity, inhibiting VEGFR3 activity is associated with the arrest of tumor vascularization, resulting in decreased vascular density in several tumor models.[1]
Since the VEGF-VEGFR pathway plays a significant role in angiogenesis, and it is widely known that VEGF is highly expressed in tumor and stromal cells, especially in the inflammatory cells of human tumors, dozens of angiogenesis inhibitors are currently undergoing clinical trials.[2] However, despite the number of compounds that has been identified for targeting the VEGF-VEGFR pathway, there is a high attrition rate. Several challenges in the development of angiogenesis inhibitors relate to their specificity, efficacy, side effects, and resistance to anti-angiogenic tumor therapy. However, the emergence of personalized medicine – based on the use of biomarkers – will likely lead to the identification of patient populations that is likely to define respondent groups.
