alleen voor onderzoeksdoeleinden
3-Methyladenine (3-MA) Autophagy/PI3K-remmer
Cat.nr.S2767
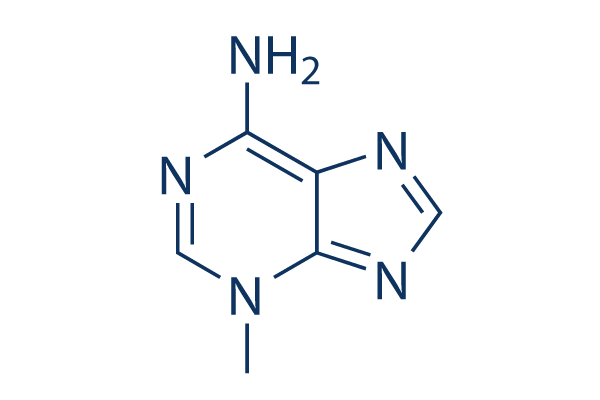
Chemische structuur
Molecuulgewicht: 149.15
Kwaliteitscontrole
Producten die vaak samen worden gebruikt met 3-Methyladenine (3-MA)
| Gerelateerde doelwitten | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Overig PI3K Inhibitoren | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Celkweek, behandeling & werkzame concentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| K562 | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| Jurkat | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| HeLa | Cytotoxicity Assay | 2mM | 24h | inhibites the cytotoxicity of silibinin to HeLa cells. | 21875385 | |
| PC12/TetOn | Function Assay | 0.1/1mM | 18h | leads to α-syn(WT) accumulation, toxicity, and oligomer formation | 21906659 | |
| RMPI8226 | Function Assay | 5mM | 1h | suppresses the level of autophagy under nutrient depletion | 21915620 | |
| MCF-7 | Function Assay | 10mM | 48h | blocks autophagy induced by bortezomib | 21931937 | |
| HBx | Apoptosis Assay | 10mM | 48h | DMSO | increases cell death | 22020078 |
| Marc-145 | Function Assay | 5mM | 12/24/36h | reduces the PRRSV titers and the protein expression | 22119900 | |
| U937 | Function Assay | 2mM | 12h | decreases the autophagy ratio | 22155150 | |
| BGC-823 | Function Assay | 5mM | 2h | inhibits the rate of autophagic cells | 22322152 | |
| A549 | Function Assay | 0.1mM | 24h | suppresses SU11274-induced cell death | 22466960 | |
| pDCs | Function Assay | 10mM | 0.5h | reduces the induction of IFN-α by ssRNA40 | 22396599 | |
| HeLa | Apoptosis Assay | 5mM | 24h | induces caspase-dependent cell death | 22545128 | |
| U251 | Apoptosis Assay | 5mM | 24h | increases S1-induced cell death | 22579788 | |
| MCF-7 | Apoptosis Assay | 0.1mM | 6h | enhances sirtinol-induced apoptosis | 22751989 | |
| PC-3 | Apoptosis Assay | 2mM | 2h | increases ORI-induced cell death | 22745580 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | DMSO | enhances apigenin-induced cell death | 24626522 |
| U2OS | Growth Inhibition Assay | 10mM | 24h | intensifies the growth inhibition induced by Dox | 24639013 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| HepG2 | Function Assay | 5mM | 4h | increases cellular levels of HL | 24713587 | |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| MDA-MB 231 | Apoptosis Assay | 5mM | 0.5h | modulates Tocomin® induced apoptosis | 24830781 | |
| PANC-1 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDA-MB-231 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MDA-MB-231 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| MCF-7 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MCF-7 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| HepG2 | Apoptosis Assay | 3mM | 5h | reduces cell apoptosis induced by QDs | 22836595 | |
| HeLa | Apoptosis Assay | 10mM | 2h | decreases cell viability co-treatment with PEI | 23000135 | |
| SK-HEP-1 | Apoptosis Assay | 10mM | 1h | protects against autophagy and induces apoptosis in bufalin-treated cells | 22858649 | |
| MDA-MB231 | Function Assay | 5mM | 1h | increases resveratrol-mediated caspase activation and cell death | 23088850 | |
| PaCa44 | Apoptosis Assay | 2.5mM | 1h | reduces genipin-mediated apoptosis | 23124112 | |
| T-47D | Function Assay | 10mM | 2h | inhibits autophagy process and increases rapamycin induced apoptosis | 23300026 | |
| GTL-16 | Apoptosis Assay | 5mM | 24h | reduces cell viability as compared to cells treated with MET inhibitors | 23313490 | |
| U251MG | Function Assay | 3mM | 1h | suppresses LC3-II protein expression | 23338618 | |
| T24 | Function Assay | 10mM | 1h | reduces the cleavage of LC3 after baicalin treatment | 23354080 | |
| HUVECs | Function Assay | 3mM | 24h | blocks the protective effect of resveratrol by inhibiting autophagy | 23358928 | |
| MCF-7 | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| Hela | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| OR6 | Function Assay | 10mM | 72h | suppresses HCV replication and formation of autophagosomes | 23395875 | |
| HT-29 | Function Assay | 1mM | 48/96h | inhibits AMPK induces autophagic cell death | 23508272 | |
| SH-SY5Y | Cytotoxicity Assay | 5mM | 24h | increases PCN toxicity | 23525265 | |
| Saos-2 | Apoptosis Assay | 1mM | 96h | increases cell death induced by PCX | 23563171 | |
| 1321N1 | Cytotoxicity Assay | 5mM | 24h | protects cell against PCN-induced toxicity | 23525265 | |
| A2780 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| OV2008 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| PC12 | Function Assay | 10mM | 24h | water | inhibits chymotrypsin-like proteasomal activity. | 23603979 |
| SH-SY5Y | Apoptosis Assay | 5mM | 1h | abolishes celastrol neuroprotective effect | 23619395 | |
| SH-SY5Y | Function Assay | 1mM | 24h | inhibits the autophagy induced by TOCP | 23743148 | |
| HepG2 | Function Assay | 10mM | 24h | inhibits siTIGAR- and HBSS-induced autophagy | 23817040 | |
| HeLa | Function Assay | 10mM | 2h | suppresses LC3 II expressison | 23864738 | |
| HONE-1 | Function Assay | 5mM | 1h | represses 6r-mediated ROS production | 23892358 | |
| MCF7 | Function Assay | 5mM | 24h | increases CuO induced cell death | 23962629 | |
| HO8910 | Apoptosis Assay | 10mM | 12h | enhances B19-induced apoptosi | 23983610 | |
| SMMC-7721 | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| Hep3B | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| H460 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| A549 | Function Assay | 10mM | 4h | inhibits autophagy induced by irradiation | 24142735 | |
| H1299 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| WiDr | Function Assay | 10mM | 1h | inhibits PCBL-induced LC3 II expression | 24190489 | |
| LoVo | Apoptosis Assay | 5mM | 48h | enhances DCA-induced apoptosis. | 24201812 | |
| HepG2 E47 | Function Assay | 2.5mM | 48h | increases the toxicity of AA, BSO, and CCl4 | 24273738 | |
| RKO | Function Assay | 2mM | 1h | DMSO | enhances cell death by geldanamycin | 24291777 |
| Hep3B | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| ACHN-5968 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| Huh7 | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| UOK257 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| ECSCs | Apoptosis Assay | 10mM | 4h | decreases rapamycin-treated apoptosis | 24319109 | |
| MCF-7 | Function Assay | 10mM | 24h | inhibits the autophagy induced by chemotherapy drugs | 24315578 | |
| SGC-7901 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| SMMC-7721 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| T24 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| NTUB1 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| MG-63 | Apoptosis Assay | 10mM | 12h | enhances DP-induced apoptosis | 24358301 | |
| MG-63 | Apoptosis Assay | 0.5/1mM | 32h | enhances salinomycin-induced cell apoptosis | 24358342 | |
| MG-63 | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| U2OS | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| HGC-27 | Function Assay | 10mM | 1h | inhibits the cell viability loss by RAD001 or MK-2206 | 24416349 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | enhances the apoptosis induced by apigenin | 24626522 | |
| A549 | Apoptosis Assay | 10mM | 48h | accelerates the reduction of cell viability induced by PTX | 24626722 | |
| Saos-2 | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| U2OS | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| HepG2 | Function Assay | 5mM | 4h | increases HL release | 24713587 | |
| A549 | Apoptosis Assay | 5mM | 48h | decreases the percentage of cell death and expression levels of caspase-3, Beclin-1 and LC3-II | 24706303 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| HT-29 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDR | Apoptosis Assay | 10mM | 6h | strengthens the power of anticancer drugs | 25019701 | |
| H157 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A549 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A2780cp | Growth Inhibition Assay | 1mM | 1h | increases cisplatin-induced cell death | 25322694 | |
| NBL-W-S | Apoptosis Assay | 1mM | 6h | increases cell apoptosis induced by GANT-61 | 25323222 | |
| NBL-W-S | Growth Inhibition Assay | 1mM | 6h | enhances GANT-61 toxicity | 25323222 | |
| A549 | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| 95D | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| A549 | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| 95D | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| Nara-H | Growth Inhibition Assay | 5mM | 48h | enhances temsirolimusmediated suppression of Nara-H cell proliferation | 21805033 | |
| HUVECs | Function Assay | 10mM | 0.5h | decreases the AGE-BSAinduced autophagy leve | 21468592 | |
| HepG2 | Apoptosis Assay | 2mM | 1h | enhances radiation-induced cell death | 21453691 | |
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Klik om meer experimentele gegevens over cellijnen te bekijken | ||||||
Chemische informatie, opslag en stabiliteit
| Molecuulgewicht | 149.15 | Formule | C6H7N5 |
Opslag (vanaf de datum van ontvangst) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS-nr. | 5142-23-4 | SDF downloaden | Opslag van stamoplossingen | Oplossingen zijn instabiel. Bereid ze vers of koop kleine, voorverpakte formaten. Herverpakken na ontvangst. | |
| Synoniemen | NSC 66389 | Smiles | CN1C=NC(=N)C2=C1N=CN2 | ||
Oplosbaarheid
|
In vitro |
DMSO
: 10 mg/mL
(67.04 mM)
Verwarmd met een waterbad van 50°C;
Geultrasoneerd;
Ethanol : 10 mg/mL Water : 4 mg/mL |
Molariteitscalculator
|
In vivo |
|||||
In vivo formulatiecalculator (heldere oplossing)
Stap 1: Voer onderstaande informatie in (Aanbevolen: een extra dier om rekening te houden met verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in de sectie oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-moedervloeistof: mg geneesmiddel vooropgelost in μL DMSO ( Concentratie moedervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de batch van het geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toeμL PEG300, mengen en verhelderen, daarna toevoegenμL Tween 80, mengen en verhelderen, daarna toevoegen μL ddH2O, mengen en verhelderen.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toe μL Maïsolie, mengen en verhelderen.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysieke methoden zoals vortexen, ultrasoon of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
Autophagy
Vps34
(HeLa cells) 25 μM
PI3Kγ
(HeLa cells) 60 μM
|
|---|---|
| In vitro |
De lichte voorkeur voor Vps34-preventie door 3-Methyladenine (3-MA) komt waarschijnlijk voort uit een hydrofobe ring die specifiek is voor Vps34 en die de 3-methylgroep van deze verbinding omgeeft. Het is gemeld dat het kankerceldood veroorzaakt onder zowel normale als uithongeringscondities, en zou ook celmigratie en invasie kunnen onderdrukken onafhankelijk van zijn vermogen om Autophagy te remmen, wat impliceert dat het functies bezit anders dan Autophagy-onderdrukking. Deze verbinding ontlokt caspase-afhankelijke celdood die onafhankelijk is van Autophagy-remming. Behandeling met 5 mM ervan vermindert het percentage glucose-uitgehongerde HeLa-cellen dat GFP-LC3-punctae vertoont tot 23%. De niveaus van LC3-I nemen toe en de niveaus van LC3-II nemen af tussen 12 en 48 uur in cellen die met 3-MA zijn behandeld. Omzetting van LC3-I naar LC3-II wordt onderdrukt door de verbinding. Behandeling van HeLa-cellen ermee bij 2,5 mM of 5 mM gedurende één dag heeft geen invloed op de cellevensvatbaarheid, terwijl behandeling met 10 mM gedurende één dag een afname van 25,0% in cellevensvatbaarheid veroorzaakt. Behandeling van cellen met 2,5, 5 of 10 mM gedurende twee dagen veroorzaakt respectievelijk 11,5%, 38,0% en 79,4% afname in levensvatbaarheid. Het vermindert de cellevensvatbaarheid op een tijd- en dosisafhankelijke manier en verkort de duur van nocodazol-geïnduceerde-prometafase-arrestatie significant. Onderdrukking van Autophagy door 3-MA remt SU11274-geïnduceerde celdood. Langdurige behandeling ermee (tot 9 uur) induceert significante LC3 I naar II conversie in wildtype MEF's. Langdurige behandeling met 3-MA, maar niet met wortmannine, verhoogt de GFP-LC3-punctatie/aggregatie aanzienlijk. De geïnduceerde LC3-conversie en vrije GFP-vrijlating zijn ATG7-afhankelijk. Behandeling ermee leidt tot een duidelijke toename van het p62-eiwitniveau. De verbinding verhoogt het p62-niveau zelfs in Atg5−/− MEF's en in cellen met DOX-gemedieerde deletie van ATG5. Het remt klasse I en klasse III PI3K in verschillende temporele patronen. De geïnduceerde LC3 I naar LC3 II conversie is dramatisch gecompromitteerd in Tsc2−/− cellen vergeleken met wildtype cellen. Deze verbinding verstoort de anti-autofagische functie van het mTOR-complex 1. |
| Kinase Assay |
Eiwitafbraaktest
|
|
HeLa-cellen worden gedurende 24 uur radiogelabeld met 0,05 mCi/mL l-[U- 14C]valine. Aan het einde van de labelingsperiode worden de cellen driemaal gespoeld met PBS. Cellen worden gedurende de aangegeven tijden geïncubeerd in ofwel volledig medium of EBSS met of zonder de aanwezigheid van 10 mM 3-Methyladenine (3-MA).
|
|
| In vivo |
3-Methyladenine (3-MA) blokkeert Autophagy via zijn effect op klasse III phosphatidylinositol 3-kinase (PI3K). Behandeling met deze verbinding verandert de mate van bloeding niet vergeleken met de subarachnoïdale bloeding (SAH) groep. De voorbehandeling ervan verergert neurologische symptomen significant in vergelijking met de SAH + voertuig groep. Autophagy is verminderd wanneer het wordt toegepast. Omgekeerd is gekliefde caspase-3 significant opgereguleerd in de SAH + 3-MA groep. In lijn met de opregulatie van gekliefde caspase-3 expressie, is het aantal TUNEL-positieve cellen in de rechter cortex significant verhoogd in de SAH + 3-MA groep vergeleken met de SAH + voertuig groep. |
Referenties |
|
Toepassingen
| Methoden | Biomarkers | Afbeeldingen | PMID |
|---|---|---|---|
| Western blot | α-SMA / TGF-β / LC-3BI / LC-3B II / Beclin-1 / NF-κB p65 caspase-3 / caspase-9 / PARP VEGF APP / BACE1 / ADAM17 / Presenilin 1 / Presenilin 2 / Nicastrin / APH-1 / Pen-2 / LC3-1 / LC3-2 |
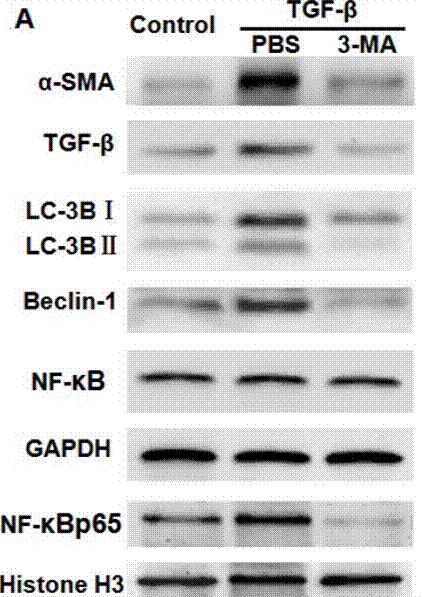
|
29296191 |
| Immunofluorescence | LC3 / Hif-α / COX2 |
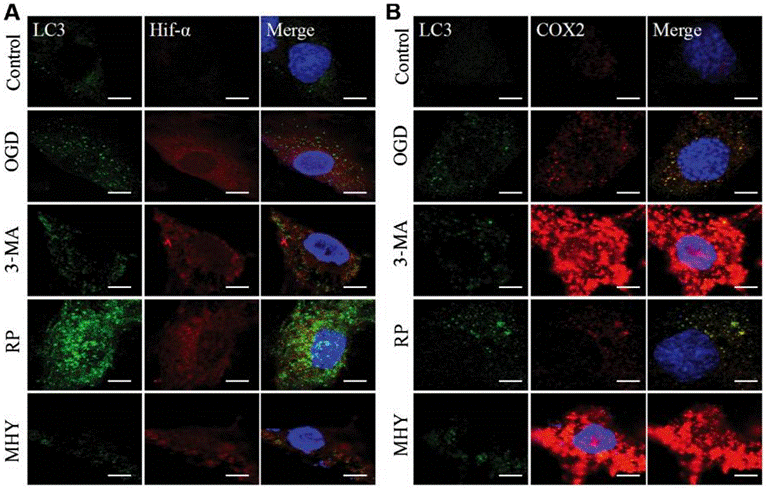
|
29039446 |
| Growth inhibition assay | Cell viability |
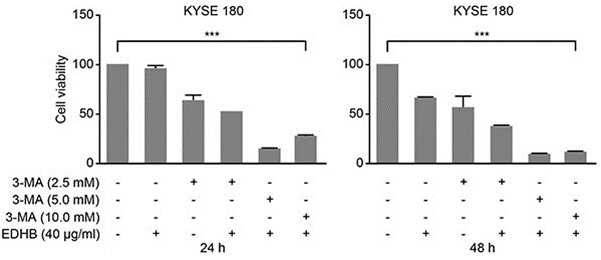
|
26934124 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, laat dan een bericht achter.
Veelgestelde vragen
Vraag 1:
I'm also wondering whether it can be dissolved in water, or maybe something like culture medium, normal saline solution to form 10mM solution.
Antwoord:
As the reference (http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal. pone.0035665), it was found to inhibit autophagy at concentrations ranging from 1 to 10 mM and was directly dissolved into the culture medium at the indicated concentrations. And we tested the solubility of S2767, and found its solubility in DMEM is 31 mg/mL at about 40°C.
