alleen voor onderzoeksdoeleinden
Tenofovir Disoproxil Fumarate Reverse Transcriptase remmer
Cat.nr.S1400
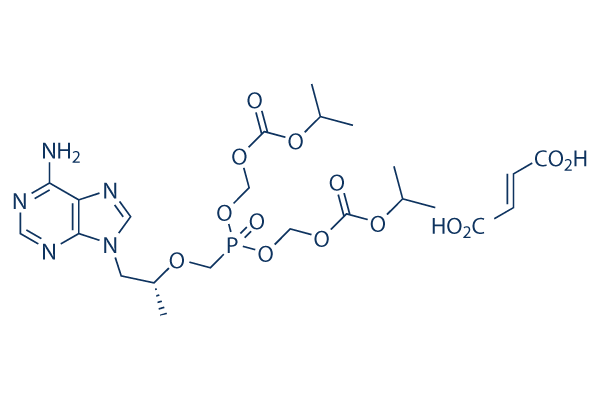
Chemische structuur
Molecuulgewicht: 635.51
Kwaliteitscontrole
| Gerelateerde doelwitten | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite HIV HCV Protease |
|---|---|
| Overig Reverse Transcriptase Inhibitoren | Dapivirine (TMC120) Fangchinoline Salicylanilide 3'-Fluoro-3'-deoxythymidine (Alovudine) Ulonivirine Lersivirine (UK-453061) Bifendate 4-Chloro-2-(trifluoroacetyl)aniline hydrochloride |
Celkweek, behandeling & werkzame concentratie
| Cellijnen | Assaytype | Concentratie | Incubatietijd | Formulering | Activiteitsbeschrijving | PMID |
|---|---|---|---|---|---|---|
| MT2 | Function assay | 5 days | Inhibition of virus-induced cytopathic effect in wild type HIV 3a infected MT2 cells after 5 days, EC50=0.015μM | 17562366 | ||
| HepG2 | Cytotoxicity assay | 9 days | Cytotoxicity against human HepG2 cells after 9 days by MTT assay, IC50=2.31μM | 17888662 | ||
| HepG2 | Antiviral assay | 9 days | Antiviral activity against Hepatitis B virus infected human HepG2 cells after 9 days by MTT assay, IC50=5.1μM | 17888662 | ||
| MT-2 | Antiviral assay | Antiviral activity against HIV1 3B infected in human MT-2 cells by two fold dilution method in presence of 10% FBS, EC50=0.0068μM | 19104010 | |||
| MT2 | Antiviral assay | Antiviral activity against HIV1 infected in human MT2 cells assessed as inhibition of viral replication, IC50=0.54μM | 19596885 | |||
| HeLa P4/R5 | Antiviral assay | Antiviral activity against HIV1 infected in human HeLa P4/R5 cells assessed as inhibition of viral replication, IC50=4.7μM | 19596885 | |||
| HeLa P4/R5 | Antiviral assay | Antiviral activity against HIV1 harboring reverse transcriptase K65R mutant infected in human HeLa P4/R5 cells assessed as inhibition of viral replication, IC50=11.4μM | 19596885 | |||
| human bone marrow cells | Cytotoxicity assay | 24 hrs | Cytotoxicity against human bone marrow cells after 24 hrs by BFU-E assay, CC50=0.9μM | 20439609 | ||
| human bone marrow cells | Cytotoxicity assay | 24 hrs | Cytotoxicity against human bone marrow cells after 24 hrs by GM-CFU assay, CC50=1.9μM | 20439609 | ||
| HeLa-T4 | Antiviral assay | 48 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef infected in human HeLa-T4 cells assessed as luciferase activity after 48 hrs by exogenous RT assay, EC50=0.0029μM | 21060108 | ||
| HeLa-T4 | Antiviral assay | 48 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef infected in human HeLa-T4 cells assessed as luciferase activity after 48 hrs by exogenous RT assay, EC95=0.037μM | 21060108 | ||
| HeLaT4 | Antiviral assay | 24 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of infection using human HeLaT4 cells pretreated for 24 hrs followed by exposed to vi, EC50=0.12μM | 21060108 | ||
| HeLaT4 | Antiviral assay | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of 2 mins magnetic nanopartials-medated infection in human HeLaT4 cells treated for 1, EC99.6=0.95μM | 21060108 | |||
| HeLaT4 | Cytotoxicity assay | Cytotoxicity against human HeLaT4 cells by WST-1 assay, CC50=34μM | 21060108 | |||
| HeLaT4 | Function assay | 24 hrs | Drug uptake in human HeLaT4 cells assessed as compound persist measured after 3 times washout at 100 time EC95 for HIV1 for 24 hrs | 21060108 | ||
| HeLaT4 | Antiviral assay | 24 hrs | Antiviral activity against single-round HIV1 NLX.Lux-R harboring inactivating mutations in env, vpr and carries firefly luciferase gene in place of nef assessed as level of infection using human HeLaT4 cells pretreated at 100 time EC95 for 24 hrs followed | 21060108 | ||
| PBMC | Antiviral assay | 7 days | Antiviral activity against HIV1 infected in human PBMC assessed as inhibition of viral replication by measuring reverse transcriptase activity in cell supernatant preincubated with cells followed by viral infection measured after 7 days by radioactive inc, EC50=0.0046μM | 27405794 | ||
| HepG2.2.15 | Antiviral assay | 3 days | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as inhibition of viral DNA in cell supernatant incubated for 3 days in presence of 10% FBS followed by compound treatment in absence of 10% FBS for 3 days by qRT-PCR method, EC50=0.34μM | 27405794 | ||
| HepG2.2.15 | Cytotoxicity assay | 6 days | Cytotoxicity against human HepG2.2.15 cells assessed as reduction in cell viability after 6 days by XTT assay, CC50=29.2μM | 27405794 | ||
| PBMC | Antiviral assay | 30 mins | Antiviral activity against CXCR4-tropic HIV-1 NL4-3 infected in human PHA-stimulated PBMC assessed as inhibition of viral replication by measuring reduction in p24 antigen production preincubated with cells for 30 mins followed by viral infection measured, IC50=0.08μM | 28682067 | ||
| PBMC | Antiviral assay | 30 mins | Antiviral activity against CCR5 tropic HIV1 BaL infected in human PHA-stimulated PBMC assessed as inhibition of viral replication by measuring reduction in p24 antigen production preincubated with cells for 30 mins followed by viral infection measured on , IC50=0.22μM | 28682067 | ||
| MT4 | Antiviral assay | 6 days | Antiviral activity against CXCR4-tropic HIV-1 NL4-3 infected in human MT4 cells assessed as inhibition of virus-induced cytopathic effect after 6 days by XTT dye based assay, EC50=4.89μM | 28682067 | ||
| MT4 | Antiviral assay | 6 days | Antiviral activity against tenofovir-resistant CXCR4-tropic HIV-1 NL4-3 harboring reverse transcriptase K65R mutant infected in human MT4 cells assessed as inhibition of viral replication inhibition of virus-induced cytopathic effect after 6 days by XTT d, EC50=11.3μM | 28682067 | ||
| HepG2.2.15 | Antiviral assay | Antiviral activity against HBV infected in human HepG2.2.15 cells assessed as reduction in cytoplasmic DNA synthesis by reed and munch method, IC50=0.85μM | 31223460 | |||
| HepG2.2.15 | Cytotoxicity assay | Cytotoxicity against human HepG2.2.15 cells infected with HBV, CC50=20.71μM | 31223460 | |||
| Klik om meer experimentele gegevens over cellijnen te bekijken | ||||||
Chemische informatie, opslag en stabiliteit
| Molecuulgewicht | 635.51 | Formule | C19H30N5O10P.C4H4O4 |
Opslag (vanaf de datum van ontvangst) | |
|---|---|---|---|---|---|
| CAS-nr. | 202138-50-9 | SDF downloaden | Opslag van stamoplossingen |
|
|
| Synoniemen | GS 1278, Tenofovir DF | Smiles | CC(C)OC(=O)OCOP(=O)(COC(C)CN1C=NC2=C(N=CN=C21)N)OCOC(=O)OC(C)C.C(=CC(=O)O)C(=O)O | ||
Oplosbaarheid
|
In vitro |
DMSO
: 100 mg/mL
(157.35 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molariteitscalculator
|
In vivo |
|||||
In vivo formulatiecalculator (heldere oplossing)
Stap 1: Voer onderstaande informatie in (Aanbevolen: een extra dier om rekening te houden met verlies tijdens het experiment)
Stap 2: Voer de in vivo formulering in (Dit is alleen de calculator, geen formulering. Neem eerst contact met ons op als er geen in vivo formulering is in de sectie oplosbaarheid.)
Berekeningsresultaten:
Werkconcentratie: mg/ml;
Methode voor het bereiden van DMSO-moedervloeistof: mg geneesmiddel vooropgelost in μL DMSO ( Concentratie moedervloeistof mg/mL, Neem eerst contact met ons op als de concentratie de DMSO-oplosbaarheid van de batch van het geneesmiddel overschrijdt. )
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toeμL PEG300, mengen en verhelderen, daarna toevoegenμL Tween 80, mengen en verhelderen, daarna toevoegen μL ddH2O, mengen en verhelderen.
Methode voor het bereiden van in vivo formulering: Neem μL DMSO moedervloeistof, voeg daarna toe μL Maïsolie, mengen en verhelderen.
Opmerking: 1. Zorg ervoor dat de vloeistof helder is voordat u het volgende oplosmiddel toevoegt.
2. Zorg ervoor dat u het/de oplosmiddel(en) in de juiste volgorde toevoegt. U moet ervoor zorgen dat de verkregen oplossing, bij de vorige toevoeging, een heldere oplossing is voordat u verdergaat met het toevoegen van het volgende oplosmiddel. Fysieke methoden zoals vortexen, ultrasoon of een warmwaterbad kunnen worden gebruikt om het oplossen te bevorderen.
Werkingsmechanisme
| Targets/IC50/Ki |
HIV reverse transcriptase
(Cell-free assay) |
|---|---|
| In vitro |
Tenofovir wordt renaal uit de systemische circulatie geëlimineerd door een combinatie van glomerulaire filtratie en actieve tubulaire secretie. Tenofovir is geen substraat voor humane organische kationentransporter type 1 (hOCT1) of hOCT2. Tenofovir accumuleert tot vijf keer lagere niveaus in MRP4-overexpresserende cellen, en de accumulatie ervan zou kunnen worden verhoogd door een MRP-remmer. Tenofovir produceert geen significante veranderingen in mitochondriale DNA (mtDNA) niveaus in humane hepatoblastoom (HepG2) cellen, skeletspiercellen (SkMCs), of renale proximale tubulus epitheelcellen. Tenofovir verhoogt de lactaatproductie met minder dan 20% in HepG2 cellen of SkMCs. Tenofovir wordt efficiënt gefosforyleerd tot tenofovirdifosfaat (TFV-DP) in zowel HepG2 cellen als primaire humane hepatocyten. Tenofovir heeft een 50% effectieve concentratie van 1,1 mM tegen HBV in celgebaseerde assays, en de potentie wordt > 50-voudig verbeterd door de toevoeging van bis-isoproxil progroepen. Tenofovir heeft eerder volledige activiteit aangetoond tegen lamivudine-resistent HBV in vitro en klinisch. Tenofovir remt de proliferatie van lever-afkomstige HepG2 cellen en normale skeletspiercellen met CC(50) waarden van respectievelijk 398 μM en 870 μM. Tenofovir toont substantieel zwakkere effecten op de proliferatie en levensvatbaarheid van renale proximale tubulus epitheelcellen dan cidofovir, een verwant nucleotide-analoog met het potentieel om renale tubulaire disfunctie te induceren. |
Referenties |
|
Informatie over klinische proeven
(gegevens van https://clinicaltrials.gov, bijgewerkt op 2024-05-22)
| NCT-nummer | Werving | Aandoeningen | Sponsor/medewerkers | Startdatum | Fasen |
|---|---|---|---|---|---|
| NCT05874440 | Recruiting | Chronic Hepatitis b Patients |
Sohag University |
April 15 2023 | -- |
| NCT03576066 | Completed | Chronic Hepatitis B |
Assembly Biosciences |
June 11 2018 | Phase 2 |
| NCT03361956 | Completed | Hepatitis B |
Janssen Sciences Ireland UC |
February 13 2018 | Phase 2 |
| NCT02985996 | Completed | HIV Infections |
Emory University|Centers for Disease Control and Prevention |
February 6 2017 | Phase 1 |
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, laat dan een bericht achter.
