Vascular Endothelial Growth Factor Receptor (VEGFR) is the receptor of VEGF. VEGFR is involved in cell proliferation, migration, survival and permeability. The VEGFs include five known structurally-related mammalian ligands (VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor, PLGF) and there are also three structurally related VEGFRs subtypes (VEGFR1, VEGFR2, and VEGFR3). [show the full text]
VEGFR inhibiteurs (VEGFR Inhibitors)
| N° de cat. | Nom du produit | Informations | Citations dutilisation du produit | Validations de produit |
|---|---|---|---|---|
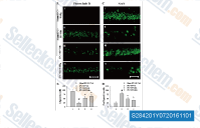
| S2842 | SAR131675 | SAR131675 est un inhibiteur de VEGFR3 avec une IC50/Ki de 23 nM/12 nM dans des tests sans cellules, environ 50 et 10 fois plus sélectif pour VEGFR3 que VEGFR1/2, peu d'activité contre Akt1, CDKs, PLK1, EGFR, IGF-1R, c-Met, Flt2 etc. |
|
|
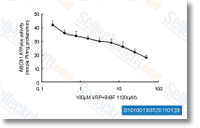
| S1010 | BIBF 1120 (Nintedanib) | Le Nintedanib est un puissant inhibiteur triple d'angiokinase pour VEGFR1/2/3, FGFR1/2/3 et PDGFRα/β avec des IC50 de 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM et 59 nM/65 nM dans des essais sans cellules. Phase 3. |
|
|
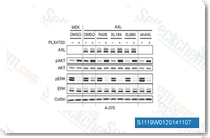
| S1119 | Cabozantinib (XL184) | Le Cabozantinib (XL184) est un puissant inhibiteur de VEGFR2 avec une IC50 de 0,035 nM et inhibe également c-Met, Ret, Kit, Flt-1/3/4, Tie2 et AXL avec une IC50 de 1,3 nM, 4 nM, 4,6 nM, 12 nM/11,3 nM/6 nM, 14,3 nM et 7 nM dans des essais acellulaires, respectivement. Il induit l'apoptose dépendante de PUMA dans les cellules de cancer du côlon via la voie de signalisation AKT/GSK-3 /NF-κB. |
|
|
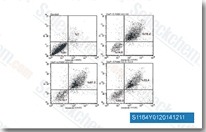
| S1164 | E7080 (Lenvatinib) | Lenvatinib est un inhibiteur multi-cibles, principalement pour VEGFR2(KDR)/VEGFR3(Flt-4) avec une IC50 de 4 nM/5.2 nM, moins puissant contre VEGFR1/Flt-1, ~10 fois plus sélectif pour VEGFR2/3 contre FGFR1, PDGFR m/ dans les essais sans cellules. Lenvatinib (E7080) inhibe également FGFR1-4, PDGFR, Kit (c-Kit), RET (c-RET), et montre de puissantes activités antitumorales. Phase 3. |
|
|
| S1005 | Axitinib (AG-013736) | Axitinib est un inhibiteur multi-cible de VEGFR1, VEGFR2, VEGFR3, PDGFR D et c-Kit avec des IC50 de 0,1 nM, 0,2 nM, 0,1-0,3 nM, 1,6 nM et 1,7 nM respectivement dans les cellules endothéliales de l'aorte porcine. |
|
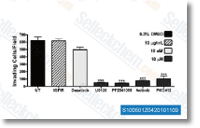
|
| S7667 | SU 5402 | SU5402 est un inhibiteur puissant et multi-ciblé de la tyrosine kinase réceptrice avec une IC50 de 20 nM, 30 nM et 510 nM pour VEGFR2, FGFR1 et PDGF-Rβ, respectivement. |
|

|
| S8401 | Erdafitinib (JNJ-42756493) | L'Erdafitinib est un inhibiteur puissant et sélectif, biodisponible par voie orale, pan-récepteur du facteur de croissance des fibroblastes (FGFR) avec une activité antinéoplasique potentielle. Ce composé se lie également à RET (c-RET), CSF-1R, PDGFR-α/PDGFR-β, FLT4, Kit (c-Kit) et VEGFR-2 et induit l'apoptose cellulaire. |
|

|
| S7397 | Sorafenib (BAY 43-9006) | Sorafenib est un inhibiteur multi-kinase de Raf-1 et B-Raf avec une IC50 de 6 nM et 22 nM respectivement dans des essais acellulaires. Sorafenib inhibe VEGFR-2, VEGFR-3, PDGFR-β, Flt-3 et c-KIT avec une IC50 de 90 nM, 20 nM, 57 nM, 59 nM et 68 nM respectivement. Sorafenib induit l'autophagy et l'apoptosis et active la ferroptosis avec une activité anti-tumorale. |
|
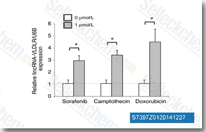
|
| S1029 | CC-5013 (Lenalidomide) | Le lénalidomide est un inhibiteur de la sécrétion de TNF-α avec une IC50 de 13 nM dans les PBMC. Le lénalidomide (CC-5013) est un ligand de l'ubiquitine E3 ligase cereblon (CRBN), et il provoque l'ubiquitination sélective et la dégradation de deux facteurs de transcription lymphoïdes, IKZF1 et IKZF3, par l'ubiquitine ligase CRBN-CRL4. Le lénalidomide favorise l'expression de la caspase-3 clivée et inhibe l'expression du VEGF et induit l'apoptose. |
|
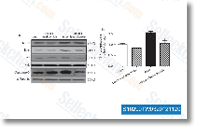
|
| S1490 | Ponatinib (AP24534) | Ponatinib est un nouvel inhibiteur multi-cible puissant de Abl, PDGFRα, VEGFR2, FGFR1 et Src avec des IC50 de 0,37 nM, 1,1 nM, 1,5 nM, 2,2 nM et 5,4 nM dans des essais sans cellules, respectivement. Ponatinib (AP24534) inhibe l'autophagy. |
|
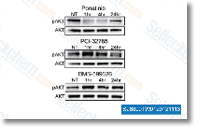
|
Solid tumors require the growth and dissemination of blood vessels and lymphatic vessels to support the metastatic growth of cancers. Following the recognition of growth factor receptor pathways that regulate angiogenesis, a number of small molecular inhibitors and antibodies have been developed that target the activity of vascular endothelial growth factor (VEGF)-VEGF receptor (VEGFR) pathway. This includes oral small-molecule tyrosine kinase inhibitors currently in clinical practice, namely sunitinib and sorafenib. These are commonly used in the treatment algorithm for renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC), two indications that are known to develop resistance to conventional chemotherapeutics.
The VEGFs include five known structurally-related mammalian ligands (VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor, PLGF). The VEGFs are disulfide-bonded homodimers, however, VEGFA and PLGF heterodimers are also known to exist. Due to alternative splicing or due to processing, VEGF ligands occur as several different variants. As a result, these variants bind differently to both VEGFRs and to co-receptors resulting in different biological responses including angiogenesis, lymphangiogenesis, permeability, inflammatory cell recruitment and fatty acid uptake. VEGFs are produced by several different cell types and act in a paracrine manner. The VEGFs bind to three structurally related tyrosine kinases (VEGFR1, VEGFR2, and VEGFR3). Modulating the effect of the VEGFRs are a number of co-receptors that lack intrinsic catalytic activity (i.e. heparin sulfate, neurophilins and integrins) and bind to VEGF.[1]
VEGFR1 (also known as Fms-like tyrosine kinase 1, Flt1, in mice) is a single-transmembrane glycoprotein structurally related to VEGFR2 and VEGFR3. VEGFR1 is expressed at high levels in vascular endothelial cells, and along with VEGFR2 binds to VEGFA. VEGFR1 is noted to bind exclusively to VEGFB and PIGF. Expression of VEGFR1 is noted to occur during vessel growth and remodeling activity. Non-endothelial cells that express VEGFR1 includes monocytes and macrophages, human tropholblasts, renal mesangial cells, vascular smooth muscle cells, dendritic cells and various tumor cells. A key regulator of VEGFR1 gene expression is hypoxia.[1]
VEGFR2 (also known as KDR; kinase insert domain receptor, in the human and Flk1; fetal liver kinase-1, in mice) binds VEGFA with a 10-fold lower affinity than VEGFR1. Other targets of VEGFR2 include proteolytically processed VEGFC and VEGFD. The only known ligand to uniquely bind to VEGFR2 is the open reading frame-encoded VEGFE. VEGFR2 is expressed in most adult vascular endothelial cells as well as circulating endothelial progenitor cells, pancreatic duct cells, retinal progenitor cells, megakaryocytes and hematopoietic cells. VEGFR2 expression is induced in conjunction with active angiogenesis (i.e. the uterus during the reproductive cycle) and in pathological process related to neovascularization (i.e. cancer). VEGFR2, often in combination with VEGFR3, is expressed at significantly upregulated levels in the tumor vascular endothelium in most common human solid tumors. Tumor cells can also express VEGFR2, however, epithelial and mesenchymal tumor cells typically express VEGFR1 rather than VEGFR2. Nevertheless, increased expression of VEGFR2 on tumor cells has been noted for melanoma and hematological malignancies. And, there is evidence supporting a relationship between chronic inflammation and tumor development.[1]
VEGFR3 (also known as Fms-like tyrosine kinase 4, Flt4 in the mouse) is activated by the binding of VEGFC or VEGFD, once these two ligands undergo proteolytic processing (this increases their affinity to VEGFR2 and VEGFR3). In addition, hVEGFD shows similar affinity to both VEGFR2 and VEGFR3, while mVEGFD binds only to VEGFR3. During embryogenesis, VEGFR3 expression occurs in the primary vascular plexus at day E8.5. In late stages of embryogenesis, VEGFR3 is expressed in venous endothelial cells of the cardinal vein, that results in VEGFR3-expressing lymphatics. Postnatally, VEGFR3 plays an important role in lymphatic endothelial cells, but its expression is also observed in endothelial cells engaged in active angiogenesis, such as tumor vessels, in endothelial tip cells of angiogenic sprouts in the developing retina or in chronic inflammatory wounds. The receptor is also found in non-endothelial cells such as osteoblasts, neuronal progenitors and macrophages – all of which may indirectly support angiogenesis. It remains unclear if tumor cells express VEGFR3. Despite this lack of clarity, inhibiting VEGFR3 activity is associated with the arrest of tumor vascularization, resulting in decreased vascular density in several tumor models.[1]
Since the VEGF-VEGFR pathway plays a significant role in angiogenesis, and it is widely known that VEGF is highly expressed in tumor and stromal cells, especially in the inflammatory cells of human tumors, dozens of angiogenesis inhibitors are currently undergoing clinical trials.[2] However, despite the number of compounds that has been identified for targeting the VEGF-VEGFR pathway, there is a high attrition rate. Several challenges in the development of angiogenesis inhibitors relate to their specificity, efficacy, side effects, and resistance to anti-angiogenic tumor therapy. However, the emergence of personalized medicine – based on the use of biomarkers – will likely lead to the identification of patient populations that is likely to define respondent groups.
