alleen voor onderzoeksgebruik
Na+/H+ Exchanger-1 Antibody [K9D3]
Cat.nr.: F4375
Gebruiksinformatie
| Verdunning |
|---|
|
| Toepassing |
|---|
| WB |
| Reactiviteit |
|---|
| Mouse, Human, Amphibian, Fish, Avian, Vertebrates |
| Bron |
|---|
| Mouse Monoclonal Antibody |
| Opslagbuffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Opslag (vanaf ontvangstdatum) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Voorspeld MW |
|---|
| ~100-110 kDa |
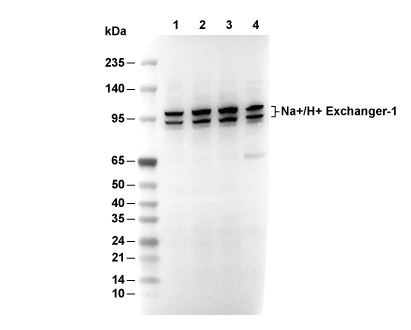
| Positieve controle | Human kidney |
|---|---|
| Negatieve controle |
Experimentele methoden
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:500), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
Biologische beschrijving
| Specificiteit |
|---|
Na+/H+ Exchanger-1 Antibody [K9D3] detects endogenous levels of total Na+/H+ Exchanger-1 protein. |
| Subcellulaire lokalisatie |
|---|
| Cell membrane, Membrane |
| Uniprot ID |
|---|
| P19634 |
| Kloon |
|---|
| K9D3 |
| Synoniem |
|---|
| Sodium/hydrogen exchanger 1; APNH; Na(+)/H(+) antiporter, amiloride-sensitive; Na(+)/H(+) exchanger 1 (NHE-1); Solute carrier family 9 member 1; SLC9A1; APNH1; NHE1 |
| Achtergrond |
|---|
Na+/H+ Exchanger-1 (NHE1, SLC9A1) is a ubiquitously expressed integral membrane antiporter that plays a central role in regulating intracellular pH (pHi) homeostasis. NHE1 is composed of 12 transmembrane helices (TMHs) with both N- and C-termini facing the cytosol. Key transport segments include TM IV, VII, and IX, along with reentrant loops IL2 and IL4, and glycosylation sites on extracellular loop 5 (EL5) that confer structural stability. The extended ~315-residue C-terminal cytoplasmic domain contains numerous serine/threonine phosphorylation sites and ezrin-binding motifs (residues 553–564), anchoring NHE1 to the actin cytoskeleton. NHE1 is allosterically activated by intracellular acidification, which promotes proton binding to a non-transport modifier site (Hill coefficient ~3), triggering conformational changes from inward- to outward-facing states through helix tilting and water-filled access pathways, similar to the bacterial NhaA transporter. It mediates electrogenic exchange of extracellular Na+ (Km 5–50 mM) for intracellular H+ in a 1:1 stoichiometry, utilizing the transmembrane Na+ gradient as its driving force without direct energy input. This allows rapid recovery of pHi following acidosis, regulation of cell volume via Na+ influx, and scaffolding of lamellipodia protrusion through ERM-actin interactions. Hormonal and growth factor signaling can phosphorylate C-terminal serines (e.g., S703, Thr653) via NHERF1, ERK, and PKA pathways, shifting the pHi set-point toward alkalinity. Pathologically, in ischemia-reperfusion injury, acid-activated NHE1 promotes cytotoxic Na+/Ca2+ overload via reverse-mode NCX activity, exacerbating myocardial infarction. Chronic NHE1 upregulation is also implicated in tumor invasion through pericellular alkalinization and matrix remodeling, as well as in cardiac hypertrophy. |
| Referenties |
|---|
|
Technische ondersteuning
Tel: +1-832-582-8158 Ext:3
Als u nog andere vragen heeft, laat dan een bericht achter.