Technische gegevens
| Formule | C23H30N8O |
||||||
| Moleculair gewicht | 434.54 | CAS-nr. | 1211441-98-3 | ||||
| Oplosbaarheid (25°C)* | In vitro | DMSO | 8 mg/mL (18.41 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Voeg oplosmiddelen afzonderlijk en in volgorde toe aan het product.) |
|
||||||
|
* <1 mg/ml betekent licht oplosbaar of onoplosbaar. * Houd er rekening mee dat Selleck de oplosbaarheid van alle verbindingen intern test en de werkelijke oplosbaarheid enigszins kan afwijken van gepubliceerde waarden. Dit is normaal en is te wijten aan lichte batch-tot-batch variaties. * Verzending op kamertemperatuur (Stabiliteitstests tonen aan dat dit product zonder koelmaatregelen kan worden verzonden.) |
|||||||
Voorbereiden van stamoplossingen
Biologische activiteit
| Beschrijving | Ribociclib is een oraal beschikbare en zeer specifieke remmer van CDK4 en CDK6 met IC50 van 10 nM en 39 nM. Fase 3. | ||||
|---|---|---|---|---|---|
| Doelen |
|
||||
| In vitro | LEE011, als duale CDK4/CDK6-remmer, remt significant de groei van 12 van de 17 neuroblastoomcellijnen met een gemiddelde IC50 van 307 nM. De groeiremming van neuroblastoomcellijnen is primair cytostatisch en wordt gemedieerd door een G1 Cell Cycle-arrest en cellulaire senescentie. |
||||
| In vivo | LEE011 (200 mg/kg dagelijks, p.o.) veroorzaakt significant tumorgroeivertraging bij muizen met de BE2C- of 1643-xenografts zonder gewichtsverlies of andere tekenen van toxiciteit. |
||||
| Kenmerken | Oraal biologisch beschikbare CDK4/6-selectieve remmer die is getest in fase III klinische proeven voor de behandeling van gevorderde borstkanker. |
Protocol (uit referentie)
| Celassay: |
|
|---|---|
| Dierstudie: |
|
Referenties
|
Klantproductvalidatie
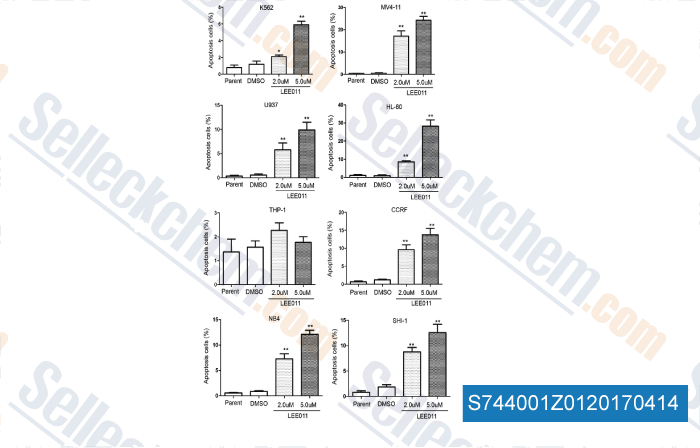
-
, , Cancer Cell Int, 2017, 17:35
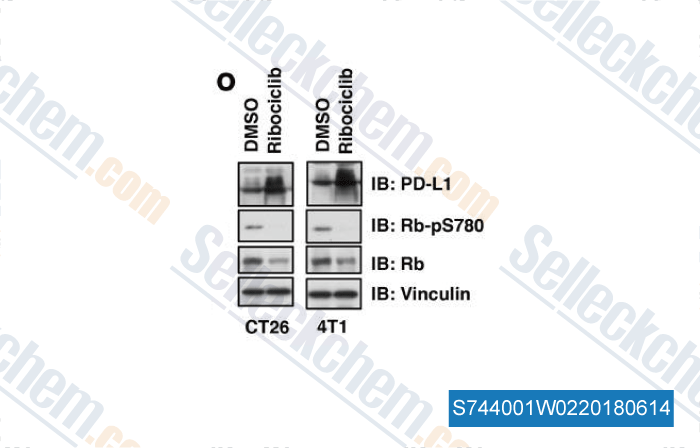
-
Gegevens van [ , , Nature, 2017, 553(7686):91-95 ]

-
Gegevens van [ , , Clin Cancer Res, 2017, 23(22):6958-6968 ]
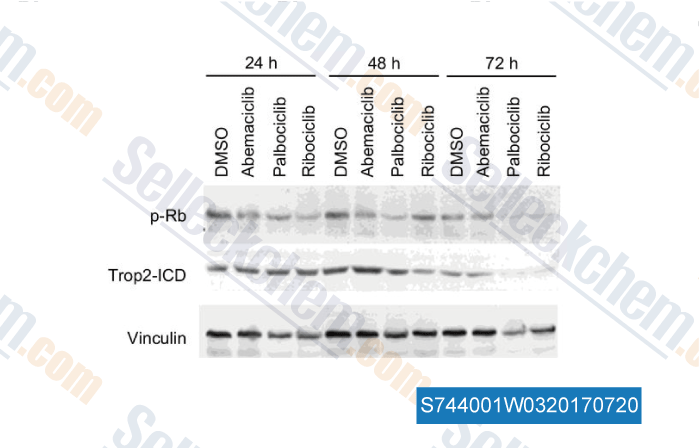
-
Gegevens van [ , , Cancer Res, 2016, 76(22):6723-6734 ]
Sellecks LEE011 (Ribociclib) Is geciteerd door 116 Publicaties
| Cellular interactions within the immune microenvironment underpins resistance to cell cycle inhibition in breast cancers [ Nat Commun, 2025, 16(1):2132] | PubMed: 40032842 |
| Combined therapy with DR5-targeting antibody-drug conjugate and CDK inhibitors as a strategy for advanced colorectal cancer [ Cell Rep Med, 2025, S2666-3791(25)00231-9] | PubMed: 40449480 |
| Resistance to CDK7 inhibitors directed by acquired mutation of a conserved residue in cancer cells [ EMBO J, 2025, 10.1038/s44318-025-00554-6] | PubMed: 40921851 |
| Blocking cancer-fibroblast mutualism inhibits proliferation of endocrine therapy resistant breast cancer [ Mol Syst Biol, 2025, 10.1038/s44320-025-00104-6] | PubMed: 40341770 |
| Gene expression analysis in circulating tumour cells to determine resistance to CDK4/6 inhibitors plus endocrine therapy in HR + /HER2- metastatic breast cancer patients [ J Transl Med, 2025, 23(1):400] | PubMed: 40186268 |
| Efficacy of CDK4/6 Inhibition in colorectal cancer and the role of p16 expression in predicting drug resistance [ Cell Oncol (Dordr), 2025, 10.1007/s13402-025-01080-7] | PubMed: 40522623 |
| FLT4 activation promotes acute lymphoid leukemia survival through stabilization of MDM2/MDMX and inactivation of p53 [ Oncogenesis, 2025, 14(1):14] | PubMed: 40316529 |
| Preclinical Evaluation of the Efficacy of a Cyclin-dependent Kinase Inhibitor Ribociclib in Combination with Letrozole Against Patient-Derived Glioblastoma Cells [ Mol Cancer Ther, 2025, 10.1158/1535-7163.MCT-25-0277] | PubMed: 40994426 |
| Investigation of ribociclib, abemaciclib and palbociclib resistance in ER+ breast cancer cells reveal potential therapeutic opportunities` [ Sci Rep, 2025, 15(1):28579] | PubMed: 40764324 |
| Bacterial ubiquitin ligase engineered for small molecule and protein target identification [ bioRxiv, 2025, 2025.03.20.644192] | PubMed: 40166235 |
RETOURBELEID
Selleck Chemicals onvoorwaardelijke retourbeleid zorgt voor een soepele online winkelervaring voor onze klanten. Als u op enigerlei wijze ontevreden bent met uw aankoop, kunt u elk artikel(en) binnen 7 dagen na ontvangst retourneren. In geval van problemen met de productkwaliteit, zowel protocolgerelateerde als productgerelateerde problemen, kunt u elk artikel(en) binnen 365 dagen na de oorspronkelijke aankoopdatum retourneren. Volg de onderstaande instructies bij het retourneren van producten.
VERZENDING EN OPSLAG
Selleck producten worden bij kamertemperatuur vervoerd. Als u het product op kamertemperatuur ontvangt, wees dan gerust, de Selleck kwaliteitsinspectieafdeling heeft experimenten uitgevoerd om te controleren of de normale temperatuurplaatsing van één maand de biologische activiteit van poederproducten niet beïnvloedt. Na ontvangst dient u het product op te slaan volgens de vereisten beschreven in het gegevensblad. De meeste Selleck producten zijn stabiel onder de aanbevolen omstandigheden.
NIET VOOR HUMANE, VETERINAIRE DIAGNOSTISCHE OF THERAPEUTISCHE DOELEINDEN.
